��Ŀ����
Ϊ��֤���Ҵ������к�����ԭ�ӣ��ֲ���һ��װ�ý���ʵ�飬�Ը�������װ���е��Լ���ʵ�����ش��й����⣺

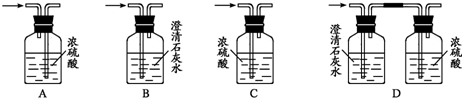
��1��װ������װ���Լ�����Aƿװ��ˮ�Ҵ����ڷ���ˮ��X��
��B�������װ��ʯ�ң�
��C��D�ж�װŨH2SO4��
��Eƿװ�Լ�Y��
��2��ʵ������������ˮԡ����Aƿ����D��ŨH2SO4��������E�����Լ�Y���ã�����C�е����д������ݷų���Aƿ��X��ɫ����B�лӷ���������ɵ�ȼ��
�ش��������⣺
��1��Eƿ����װ���Լ�Y��____________��
�ٱ���ʳ��ˮ ��MnO2��NaCl�Ļ���� ��ŨHCl
��2��D��ŨH2SO4�����������_________________��C��ŨH2SO4�����������__________��
��3��Aƿ�з�����Ӧ�Ļ�ѧ����ʽ��____________________����Ӧ������___________�������ɵ�_________��д���ƣ���B���ڴ���ȼ��
��4����ˮ��X��ѡ��__________��������ָʾ�����õ�ԭ����____________________��
��5����ʵ����֤���Ҵ������к�����ԭ�ӵ�������____________________��
��6�������װ���е�Cƿȥ���ܷ�ó����ս��ۣ�____________________��Ϊʲô��
��1���ۣ�2������ŨHCl�е�ˮ�֣�ʹHCl�����ݳ�������HCl���壨3��CH3CH2OH+HCl![]() CH3CH2Cl+H2O��ȡ����Ӧ�������飨4����ˮCuSO4��ʵ������й۲쵽��ˮCuSO4������֤����Ӧ��ˮ���ɣ�5����ˮCuSO4����֤����ˮ���ɣ�ˮ�к���Ԫ�أ�������������HCl��ֻ�������Ҵ���6�����ܣ�������ȥˮ�����ж�ʹ��ˮCuSO4������ˮ�Ƿ��������Ҵ���HCl�ķ�Ӧ��
CH3CH2Cl+H2O��ȡ����Ӧ�������飨4����ˮCuSO4��ʵ������й۲쵽��ˮCuSO4������֤����Ӧ��ˮ���ɣ�5����ˮCuSO4����֤����ˮ���ɣ�ˮ�к���Ԫ�أ�������������HCl��ֻ�������Ҵ���6�����ܣ�������ȥˮ�����ж�ʹ��ˮCuSO4������ˮ�Ƿ��������Ҵ���HCl�ķ�Ӧ��
����: