��Ŀ����
��Դ��������ᷢչ�Ļ��������������������Դ��̫���ܣ�����������Դ��ֲ�P��ӹ���Ʒ���������������ʯȼ���̲ص�����Ҳ����Զ��ʱ�����������������õ�̫���ܣ�
��1���ڵ������ǵ������У�����֪��������̫���ܷ����������ķ�ʽ��
��2�����������ܵ����ù����У������Ƶ���Ҫ���л����Ҵ���ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ������Ԫ�ص�ȷ��������ʽ��ȷ�������ӽṹ��ȷ����
�������Ǿ����÷����Ҵ�ȼ�յIJ�����ȷ���Ҵ��к���C��H����Ԫ�أ���Ҫ˵�����ǵ�ʵ���������������
��֤��������Ԫ�ص�ʵ�����������������
��֤������̼Ԫ�ص�ʵ�����������������
�����÷���ȼ�ղ�����֤ʵ�Ҵ��л�������Ԫ��ʱ����Ҫȡ��һЩʵ�����ݣ���Щ����Ӧ����
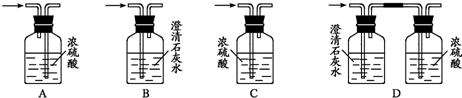
ʵ�������ǿ��ܻ��õ�����װ�ã����㽫��������װ�õ���Ű��������ų�������˳����ʾ���������
����Ϊȷ���Ҵ��ķ���ʽ������������Ҫȡ�õ������⣬����Ϊ���Ƿ���Ҫ�ⶨ�Ҵ�����Է��������أ�
������Ϊȷ���Ҵ��ķ��ӽṹ�������Ȳⶨһ������ˮ�Ҵ��ͽ����Ʒ�Ӧ���������������ѡ��������ͼ��ʾ������װ�ã��е���������˫����Ƥ��������
����Щ����װ�õĺ���������˳����
����ʵ��֤���Ҵ��ķ��ӽṹ��CH3CH2OH������CH3OCH3��������
��3����������Դ�Ի���ʯ�͵ȵĶ�ȱ�������ԴΣ����һ����Ҫ���⣮�ݱ�����ij�ؽ��꽨����һ����������ҵ�ƾ�500��ֵĹ�����Ŀ����Ϊ�˽���ҵ�ƾ������ͻ����Ϊ����ȼ�϶��������͵�����������֪�����ƾ��ķ������������֣��û�ѧ����ʽ��ʾ����
����һ��CH2=CH2+H2O
CH3CH2OH
��������CH3-CH2Br+H2O
CH3CH2OH+HBr
����������C6H10O5��n�����ۣ�+n H2O
n C6H12O6�������ǣ�
C6H12O6�������ǣ�
2C2H5OH+2CO2��
�ۺϵؿ�������Ϊ�����ַ����У��Ϻõ���
A������һ B�������� C��������
����Ϊ�ù���������ҵ�ƾ��õ���
��1���ڵ������ǵ������У�����֪��������̫���ܷ����������ķ�ʽ��
��Ĥ��������ҡ�̫������ˮ����
��Ĥ��������ҡ�̫������ˮ����
���ξ�����������2�����������ܵ����ù����У������Ƶ���Ҫ���л����Ҵ���ijУ��ѧ������ȤС���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ������Ԫ�ص�ȷ��������ʽ��ȷ�������ӽṹ��ȷ����
�������Ǿ����÷����Ҵ�ȼ�յIJ�����ȷ���Ҵ��к���C��H����Ԫ�أ���Ҫ˵�����ǵ�ʵ���������������
��֤��������Ԫ�ص�ʵ�����������������
��һ�����С�ձ��������Ҵ�ȼ�ջ����Ϸ����ձ��ڱ���ˮ������
��һ�����С�ձ��������Ҵ�ȼ�ջ����Ϸ����ձ��ڱ���ˮ������
����֤������̼Ԫ�ص�ʵ�����������������
���ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ����Ϸ���ʯ��ˮ�����
���ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ����Ϸ���ʯ��ˮ�����
�������÷���ȼ�ղ�����֤ʵ�Ҵ��л�������Ԫ��ʱ����Ҫȡ��һЩʵ�����ݣ���Щ����Ӧ����
�Ҵ���������̼��ˮ�������ʵ�����
�Ҵ���������̼��ˮ�������ʵ�����
��ʵ�������ǿ��ܻ��õ�����װ�ã����㽫��������װ�õ���Ű��������ų�������˳����ʾ���������
CD��CB
CD��CB
��
����Ϊȷ���Ҵ��ķ���ʽ������������Ҫȡ�õ������⣬����Ϊ���Ƿ���Ҫ�ⶨ�Ҵ�����Է��������أ�
����Ҫ
����Ҫ
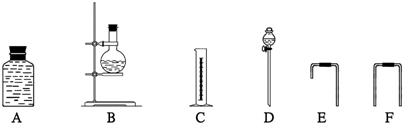
��������Ϊȷ���Ҵ��ķ��ӽṹ�������Ȳⶨһ������ˮ�Ҵ��ͽ����Ʒ�Ӧ���������������ѡ��������ͼ��ʾ������װ�ã��е���������˫����Ƥ��������

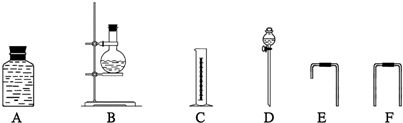
����Щ����װ�õĺ���������˳����
D
D
��B
B
��E
E
��A
A
��F
F
��C
C
�����������������װ�õĴ�д��ĸ��������ʵ��֤���Ҵ��ķ��ӽṹ��CH3CH2OH������CH3OCH3��������
����Na���Ҵ���Ӧ���ɵ�H2�����жϳ�ֻ��һ��H������Hԭ�Ӵ��ڲ�ͬ������λ�ã��Ӷ���һ��ȷ����ṹΪC2H5OH����CH3-O-CH3
����Na���Ҵ���Ӧ���ɵ�H2�����жϳ�ֻ��һ��H������Hԭ�Ӵ��ڲ�ͬ������λ�ã��Ӷ���һ��ȷ����ṹΪC2H5OH����CH3-O-CH3
����3����������Դ�Ի���ʯ�͵ȵĶ�ȱ�������ԴΣ����һ����Ҫ���⣮�ݱ�����ij�ؽ��꽨����һ����������ҵ�ƾ�500��ֵĹ�����Ŀ����Ϊ�˽���ҵ�ƾ������ͻ����Ϊ����ȼ�϶��������͵�����������֪�����ƾ��ķ������������֣��û�ѧ����ʽ��ʾ����
����һ��CH2=CH2+H2O
| ||
| �� |
��������CH3-CH2Br+H2O
| ||
| �� |
����������C6H10O5��n�����ۣ�+n H2O
| ����ø |
C6H12O6�������ǣ�
| �ƻ�ø |
�ۺϵؿ�������Ϊ�����ַ����У��Ϻõ���
A
A
������ĸ������������Ӧԭ��������ɫ��ѧ��ԭ��Ӧ���ԭ��ȫ��ת��Ϊ���������ղ���
��Ӧԭ��������ɫ��ѧ��ԭ��Ӧ���ԭ��ȫ��ת��Ϊ���������ղ���
��A������һ B�������� C��������
����Ϊ�ù���������ҵ�ƾ��õ���
������
������
�������һ��������������������������������������ַ�����ԭ������ϩ�������鶼����������ʯ��Ϊԭ���Ƶõ����ʣ�ʯ���Dz��������ģ����������Ƴɾƾ�������ֱ������ʯ��
��ϩ�������鶼����������ʯ��Ϊԭ���Ƶõ����ʣ�ʯ���Dz��������ģ����������Ƴɾƾ�������ֱ������ʯ��
_����������1����-����ת��ԭ����ֱ������̫��������������������ܣ��磺����ʽ̫�������̫��¯����Ĥ��������ҡ�̫������ˮ���ȣ�
��2��������һ�����С�ձ��������Ҵ�ȼ�ջ����Ϸ����ձ��ڱ���ˮ�����ɣ�֤����Ϊ����HԪ�أ�
�ڽ��ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ����Ϸ���ʯ��ˮ����ǣ�֤����Ϊ����CԪ�أ�
�����Ҵ���ȫȼ�պ����ֻ��CO2��H2O�����������غ㶨�ɿ�֪��������һ������̼����Ԫ�أ���Ԫ�ز���ȷ������ͨ���ⶨ�л��PCO2��H2O����������C��H��Ԫ�ص�����֮�͵����л��������������л���ֻ��C��H��Ԫ�أ���C��H��Ԫ�ص�����֮��С���л��������������л���һ������C��H��O����Ԫ�أ�
����Ũ��������أ��ⶨ����ˮ�����������ݳ���ʯ��ˮ���أ�������̼����������ⶨ������̼������
�����ݾ���Cԭ�ӡ�Hԭ�ӡ�Oԭ�Ӹ������жϣ�
��������1���ٸ���ʵ��ԭ����������װ�õ����ý�����������
�ڸ��ݷ���ʽд�����ܽṹ�����Na���Ҵ���Ӧ���ɵ�H2�����жϣ�
��3�����ݷ�Ӧԭ��������ɫ��ѧ��ԭ��ԭ���ǿ����������ʣ�������á�����������
��2��������һ�����С�ձ��������Ҵ�ȼ�ջ����Ϸ����ձ��ڱ���ˮ�����ɣ�֤����Ϊ����HԪ�أ�
�ڽ��ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ����Ϸ���ʯ��ˮ����ǣ�֤����Ϊ����CԪ�أ�
�����Ҵ���ȫȼ�պ����ֻ��CO2��H2O�����������غ㶨�ɿ�֪��������һ������̼����Ԫ�أ���Ԫ�ز���ȷ������ͨ���ⶨ�л��PCO2��H2O����������C��H��Ԫ�ص�����֮�͵����л��������������л���ֻ��C��H��Ԫ�أ���C��H��Ԫ�ص�����֮��С���л��������������л���һ������C��H��O����Ԫ�أ�
����Ũ��������أ��ⶨ����ˮ�����������ݳ���ʯ��ˮ���أ�������̼����������ⶨ������̼������
�����ݾ���Cԭ�ӡ�Hԭ�ӡ�Oԭ�Ӹ������жϣ�
��������1���ٸ���ʵ��ԭ����������װ�õ����ý�����������
�ڸ��ݷ���ʽд�����ܽṹ�����Na���Ҵ���Ӧ���ɵ�H2�����жϣ�
��3�����ݷ�Ӧԭ��������ɫ��ѧ��ԭ��ԭ���ǿ����������ʣ�������á�����������
����⣺��1��ֱ������̫��������������������ܵ�Ӧ�úܶ࣬�磺����ʽ̫�������̫��¯����Ĥ��������ҡ�̫������ˮ���ȣ�
�ʴ�Ϊ����Ĥ��������ҡ�̫������ˮ���ȣ�
��2��������һ�����С�ձ��������Ҵ�ȼ�ջ����Ϸ����ձ��ڱ���ˮ�����ɣ�֤����Ϊ����HԪ�أ�
�ʴ�Ϊ����һ�����С�ձ��������Ҵ�ȼ�ջ����Ϸ����ձ��ڱ���ˮ�����ɣ�
�ڽ��ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ����Ϸ���ʯ��ˮ����ǣ�֤����Ϊ����CԪ�أ�
�ʴ�Ϊ�����ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ����Ϸ���ʯ��ˮ����ǣ�
����ͨ���ⶨ�Ҵ���CO2��H2O����������C��H��Ԫ�ص�����֮�͵����Ҵ�������������л���ֻ��C��H��Ԫ�أ�
��C��H��Ԫ�ص�����֮��С���Ҵ�������������л���һ������C��H��O����Ԫ�أ�
A���������������ʴ������ų�������˳��CD��CB��
�ʴ�Ϊ���Ҵ���������̼��ˮ�������ʵ�������CD��CB��
�������Ҵ������У�C��Hԭ���Ա��ͣ�ʵ��ʽ��Ϊ����ʽ���ʲ���Ҫ�ⶨ�Ҵ�����Է���������
�ʴ�Ϊ������Ҫ��
������ ��D��B���Ϊ����װ�ã�ͨ��D�ɱ�����B�����Ӿƾ���A��Cͨ��F���ӹ�������װ�ã���װ��ͨ��E���ӣ�������˳��ΪD��B��E��A��F��C��
�ʴ�Ϊ��D��B��E��A��F��C��
�ڸ���Na���Ҵ���Ӧ���ɵ�H2�����жϳ�ֻ��һ��H������Hԭ�Ӵ��ڲ�ͬ������λ�ã��Ӷ���һ��ȷ����ṹΪC2H5OH����CH3-O-CH3��
�ʴ�Ϊ������Na���Ҵ���Ӧ���ɵ�H2�����жϳ�ֻ��һ��H������Hԭ�Ӵ��ڲ�ͬ������λ�ã��Ӷ���һ��ȷ����ṹΪ
C2H5OH����CH3-O-CH3��
��3��A��Ӧԭ��������ɫ��ѧ��ԭ��Ӧ���ԭ��ȫ��ת��Ϊ���������ղ������B ԭ���ǿ����������ʣ�������á���������
�����ǿ����������ʣ�������á���������ϩ�������鶼����������ʯ��Ϊԭ���Ƶõ����ʣ�ʯ���Dz��������ģ����������Ƴɾƾ�������ֱ������ʯ�ͣ�
�ʴ�Ϊ��A����Ӧԭ��������ɫ��ѧ��ԭ��Ӧ���ԭ��ȫ��ת��Ϊ���������ղ����B��ԭ���ǿ����������ʣ�������á�������������������ϩ�������鶼����������ʯ��Ϊԭ���Ƶõ����ʣ�ʯ���Dz��������ģ����������Ƴɾƾ�������ֱ������ʯ�ͣ�
�ʴ�Ϊ����Ĥ��������ҡ�̫������ˮ���ȣ�
��2��������һ�����С�ձ��������Ҵ�ȼ�ջ����Ϸ����ձ��ڱ���ˮ�����ɣ�֤����Ϊ����HԪ�أ�
�ʴ�Ϊ����һ�����С�ձ��������Ҵ�ȼ�ջ����Ϸ����ձ��ڱ���ˮ�����ɣ�
�ڽ��ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ����Ϸ���ʯ��ˮ����ǣ�֤����Ϊ����CԪ�أ�
�ʴ�Ϊ�����ڱ��ó���ʯ��ˮ��ʪ��С�ձ������ڻ����Ϸ���ʯ��ˮ����ǣ�
����ͨ���ⶨ�Ҵ���CO2��H2O����������C��H��Ԫ�ص�����֮�͵����Ҵ�������������л���ֻ��C��H��Ԫ�أ�
��C��H��Ԫ�ص�����֮��С���Ҵ�������������л���һ������C��H��O����Ԫ�أ�
A���������������ʴ������ų�������˳��CD��CB��
�ʴ�Ϊ���Ҵ���������̼��ˮ�������ʵ�������CD��CB��
�������Ҵ������У�C��Hԭ���Ա��ͣ�ʵ��ʽ��Ϊ����ʽ���ʲ���Ҫ�ⶨ�Ҵ�����Է���������
�ʴ�Ϊ������Ҫ��
������ ��D��B���Ϊ����װ�ã�ͨ��D�ɱ�����B�����Ӿƾ���A��Cͨ��F���ӹ�������װ�ã���װ��ͨ��E���ӣ�������˳��ΪD��B��E��A��F��C��
�ʴ�Ϊ��D��B��E��A��F��C��
�ڸ���Na���Ҵ���Ӧ���ɵ�H2�����жϳ�ֻ��һ��H������Hԭ�Ӵ��ڲ�ͬ������λ�ã��Ӷ���һ��ȷ����ṹΪC2H5OH����CH3-O-CH3��
�ʴ�Ϊ������Na���Ҵ���Ӧ���ɵ�H2�����жϳ�ֻ��һ��H������Hԭ�Ӵ��ڲ�ͬ������λ�ã��Ӷ���һ��ȷ����ṹΪ
C2H5OH����CH3-O-CH3��
��3��A��Ӧԭ��������ɫ��ѧ��ԭ��Ӧ���ԭ��ȫ��ת��Ϊ���������ղ������B ԭ���ǿ����������ʣ�������á���������
�����ǿ����������ʣ�������á���������ϩ�������鶼����������ʯ��Ϊԭ���Ƶõ����ʣ�ʯ���Dz��������ģ����������Ƴɾƾ�������ֱ������ʯ�ͣ�
�ʴ�Ϊ��A����Ӧԭ��������ɫ��ѧ��ԭ��Ӧ���ԭ��ȫ��ת��Ϊ���������ղ����B��ԭ���ǿ����������ʣ�������á�������������������ϩ�������鶼����������ʯ��Ϊԭ���Ƶõ����ʣ�ʯ���Dz��������ģ����������Ƴɾƾ�������ֱ������ʯ�ͣ�
�����������л������ʽ���ṹʽ��ȷ�����Ѷ��еȣ�����ȼ�շ���������غ㶨��ȷ��ʵ��ʽ���ٽ�������ƶ���ṹ����ѧ�������ۺ��Կ��飮

��ϰ��ϵ�д�
�����Ŀ



 CH3CH2OH
CH3CH2OH CH3CH2OH+HBr
CH3CH2OH+HBr n C6H12O6�������ǣ�
n C6H12O6�������ǣ� 2C2H5OH+2CO2��
2C2H5OH+2CO2��