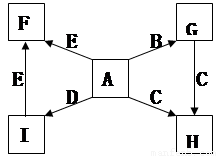
��Ŀ����
��ÿ��2�֣���14�֣�A��B��C��D��E��F������ѧ��ѧ�г��������ʣ�����ͼת����ϵ����

��1����A��C��E��Ϊ�������EΪ�ж����壬B��D��F��Ϊ�ǽ������ʣ���DΪ���塣��A�Ľṹ��ʽΪ ������F�ľ�������Ϊ ��1molC�к��� �����ۼ���
��2����A��C��E��Ϊ�⻯����߽�Ϊ���Է��ӣ�����C����Է���������С��D��E��F��Ϊ����� ����D��FΪ�ж����壬F��������Ϊ����ɫ�� ��
��A��C��E�е��ɸߵ��͵�˳��Ϊ �����ѧʽ����A��C��E������̬�⻯���ȶ�����ǿ���� ���ѧʽ����
��B��C��Ӧ����E��F�Ļ�ѧ����ʽΪ ��
��A��D�����·�Ӧ������һ�ֵ��ʣ���ѧ����ʽΪ ��
��ÿ��2�֣���14�֣���1��H-O-H��ԭ�Ӿ��壬4NA����2����H2O��NH3��H2S��H2O��
��4NH3 + 5O2
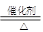 4NO +6 H2O�� ��SO2 + 2H2S��2H2O + 3S��
4NO +6 H2O�� ��SO2 + 2H2S��2H2O + 3S��
����������1���������ⷴӦ�����û���Ӧ������EΪ�ж����壬����EˮCO����A��ˮ��B��̼��C�Ƕ������裬D��������F�ǹ衣�ڶ������辧�壬ÿ����ԭ���γ�4��������������1mol���������к���4NA�����ۼ���
��2��D��FΪ�ж����壬F��������Ϊ����ɫ����F��NO��C�ǰ�����B��������E��ˮ��A�����⣬D��SO2��
��ˮ���Ӽ䡢�������Ӽ䶼����������Էе㶼��������ģ���H2O��NH3��H2S���ǽ�����Խǿ���⻯����ȶ���Խǿ��ˮ���ȶ���
�ڸ÷�Ӧ�ǰ��Ĵ�����������ʽΪ4NH3 + 5O2 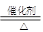 4NO
+6 H2O��
4NO
+6 H2O��
��SO2���������ԣ��ܺ������������ɵ������ˮ������ʽΪSO2 + 2H2S��2H2O + 3S����

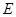 ���Է�����Ӧ��2E��I
���Է�����Ӧ��2E��I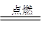 2F��D��F�е�EԪ�ص���������Ϊ60%.
2F��D��F�е�EԪ�ص���������Ϊ60%.