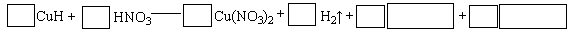��Ŀ����
��֪����ǿ�������Һ���ֱ������������������еĸ�һ�֣��һ����ظ���NH4����Ba2����Na����H����SO42����NO3����OH����CO32��������������Һ�ֱ���ΪA��B��C��D����������ʵ�飺
����A��D�е���C�����г������ɣ���D��B��Ӧ���ɵ������ܱ�A���գ���A��D��Ӧ���ɵ������ܱ�B���ա��Իش��������⣺
(1)D�Ļ�ѧʽ�� ���ж������� ��
(2)д�����༸�����ʵĻ�ѧʽ��A ��B ��C ��
(3)д��ʵ������йط�Ӧ�����ӷ���ʽ �� ��
����A��D�е���C�����г������ɣ���D��B��Ӧ���ɵ������ܱ�A���գ���A��D��Ӧ���ɵ������ܱ�B���ա��Իش��������⣺
(1)D�Ļ�ѧʽ�� ���ж������� ��
(2)д�����༸�����ʵĻ�ѧʽ��A ��B ��C ��
(3)д��ʵ������йط�Ӧ�����ӷ���ʽ �� ��
(1)(NH4)2CO3 D��A��B�����������壬�����������ֻ��H����CO32����OH����NH4���ܷ�Ӧ�������壬Dֻ��Ϊ(NH4)2CO3 ÿ��2��
(2)H2SO4 NaOH Ba(NO3)2 ÿ��2��
(3)NH4����OH�� NH3����H2O�� NH3��H��=NH4�� ÿ������ʽ2��
NH3����H2O�� NH3��H��=NH4�� ÿ������ʽ2��
(2)H2SO4 NaOH Ba(NO3)2 ÿ��2��
(3)NH4����OH��
 NH3����H2O�� NH3��H��=NH4�� ÿ������ʽ2��
NH3����H2O�� NH3��H��=NH4�� ÿ������ʽ2�������������������֪�������ݢٿ�֪A��D�к�SO42����CO32��һ�֣�C�к�Ba2�������ݢڢۿ�֪D��A��B�����������壬�����������ֻ��H����CO32����OH����NH4���ܷ�Ӧ�������壬����Dֻ��Ϊ(NH4)2CO3��A�к�SO42��Ҳ��ֻ����H2SO4��CΪ��Һ���ֺ���Ba2�����ӵ�ֻ����Ba(NO3)2 ��ʣ�����ӽ��ΪNaOH����B ��ΪNaOH.ʵ������йط�Ӧ�����ӷ���ʽ���ԡ�

��ϰ��ϵ�д�
 ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ

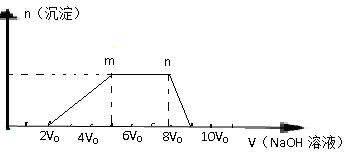
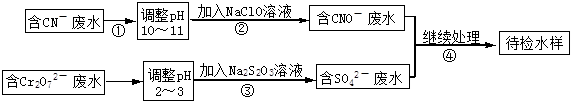 �ش��������⣺
�ش��������⣺