��Ŀ����
��֪2SO2(g)��O2(g)  2SO3(g)����H����a kJ��mol��1(a>0)�����º����£���10 L���ܱ������м���0.1 mol SO2��0.05 mol O2������2 min�ﵽƽ��״̬����Ӧ����0.025 a kJ�������ж���ȷ����(����)
2SO3(g)����H����a kJ��mol��1(a>0)�����º����£���10 L���ܱ������м���0.1 mol SO2��0.05 mol O2������2 min�ﵽƽ��״̬����Ӧ����0.025 a kJ�������ж���ȷ����(����)
 2SO3(g)����H����a kJ��mol��1(a>0)�����º����£���10 L���ܱ������м���0.1 mol SO2��0.05 mol O2������2 min�ﵽƽ��״̬����Ӧ����0.025 a kJ�������ж���ȷ����(����)
2SO3(g)����H����a kJ��mol��1(a>0)�����º����£���10 L���ܱ������м���0.1 mol SO2��0.05 mol O2������2 min�ﵽƽ��״̬����Ӧ����0.025 a kJ�������ж���ȷ����(����)| A����2 min�ڣ�v(SO2)��0.25 mol��L��1��min��1 |
| B�����ٳ���0.1 mol SO3���ﵽƽ���SO3�������������С |
| C����1 minʱ��c(SO2)��c(SO3)��0.01 mol��L��1 |
| D�������º�ѹ�£���10 L���ܱ������м���0.1 mol SO2��0.05 mol O2��ƽ���Ӧ����С��0.025a kJ |
C
A���2 min�ڣ�v(SO2)��0.002 5 mol��L��1��min��1��B����ٳ���0.1 mol SO3����ͬ�ڡ��ٳ���0.1 mol SO2��0.05 mol O2������ʱ�൱�ڼ�ѹ����Ӧ��ƽ��ת������ߣ��ﵽƽ���SO3��������������C�������ԭ���غ㣬�÷�Ӧ��ϵ���κ�ʱ�̶���c(SO2)��c(SO3)��0.01 mol��L��1��D�2SO2(g)��O2(g)??2SO3(g)������Ӧ��һ���������ʵ�����С�ķ�Ӧ���ں��º��������£����ŷ�Ӧ������У������ڵ�ѹǿ��С�������º�ѹ���������൱����ԭ���º��ݷ�Ӧ��ϵ�Ļ����ϼ�ѹ����Ӧ���ƽ��ת������ߣ�ƽ���Ӧ���ȴ���0.025a kJ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
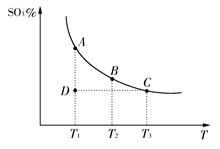
 2SO3(g)�������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ(�������κ�һ�㶼��ʾƽ��״̬)������ͼʾ�ش��������⣺
2SO3(g)�������ϵ��SO3�İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ(�������κ�һ�㶼��ʾƽ��״̬)������ͼʾ�ش��������⣺ 2SO3�ķ�Ӧ��������������ȷ���ǣ� ��
2SO3�ķ�Ӧ��������������ȷ���ǣ� �� CH3CH2OH(g)+H2O(g)�����ܱ������г���10 mol CO��20mol H2���ڴ��������·�Ӧ�����Ҵ���CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3CH2OH(g)+H2O(g)�����ܱ������г���10 mol CO��20mol H2���ڴ��������·�Ӧ�����Ҵ���CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
 CH3OH(g)��H2O(g)������6mol CO2��8 mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���
CH3OH(g)��H2O(g)������6mol CO2��8 mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���
 CH3OH(g)��
CH3OH(g)��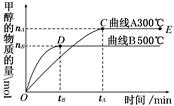
 H2(g)+CO2(g) ��H="-41.2" kJ/mol�Ʊ�CO2��H2�Ļ������,����һ���о�CO2��H2�Բ�ͬ������Ȼ��ʱ�ں��������µķ�Ӧ����Ӧ�á�
H2(g)+CO2(g) ��H="-41.2" kJ/mol�Ʊ�CO2��H2�Ļ������,����һ���о�CO2��H2�Բ�ͬ������Ȼ��ʱ�ں��������µķ�Ӧ����Ӧ�á�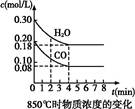
 pC(g)+qD(g)ƽ�ⳣ��ΪK������˵����ȷ����( )
pC(g)+qD(g)ƽ�ⳣ��ΪK������˵����ȷ����( ) 2C(g)��һ��ʱ���ﵽƽ�⣬����n mol C��������˵������ȷ����(����)
2C(g)��һ��ʱ���ﵽƽ�⣬����n mol C��������˵������ȷ����(����)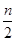 ��
�� C(g)��D(g)�Ѵﵽƽ��״̬( )
C(g)��D(g)�Ѵﵽƽ��״̬( )