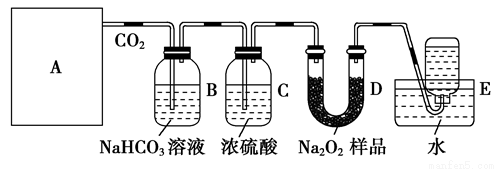
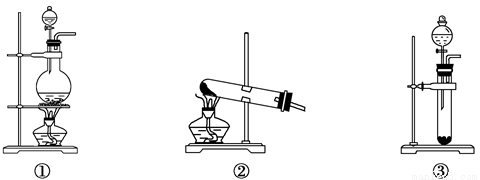
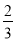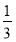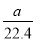ΧβΡΩΡΎ»ί
Β―ι “–η“Σ2.0 molΓΛL-1NaOH»ή“Κ90mLΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©≈δ÷ΤΙΐ≥Χ÷–≤Μ–η“Σ Ι”ΟΒΫΒΡΜ·―ß“«Τς”– Θ®ΧνΉ÷ΡΗΘ©
A.…’±≠ B. 100mL»ίΝΩΤΩ C. ¬©ΕΖ D.ΫΚΆΖΒΈΙή E.≤ΘΝßΑτ
Θ®2Θ©”ΟΆ–≈ΧΧλΤΫ≥Τ»Γ«β―θΜ·ΡΤΘ§Τδ÷ ΝΩΈΣ gΘΜ
Θ®3Θ©œ¬Ν–÷ς“Σ≤ΌΉς≤Ϋ÷ηΒΡ’ΐ»ΖΥ≥–ρ « Θ®Χν–ρΚ≈Θ©ΘΜ
ΔΌ≥Τ»Γ“ΜΕ®÷ ΝΩΒΡ«β―θΜ·ΡΤΘ§Ζ≈»κ…’±≠÷–Θ§”Ο ΝΩ’τΝσΥ°»ήΫβΘΜ
ΔΎΦ”Υ°÷Ν“ΚΟφάκ»ίΝΩΤΩΨ±ΩΧΕ»œΏœ¬1ΓΣ2άεΟΉ ±Θ§ΗΡ”ΟΫΚΆΖΒΈΙήΒΈΦ”’τΝσΥ°÷ΝΑΦ“ΚΟφ”κΩΧΕ»œΏœύ«–ΘΜ
Δέ¥ΐά以÷Ν “Έ¬ΚσΘ§ΫΪ»ή“ΚΉΣ“ΤΒΫ100mL»ίΝΩΤΩ÷–ΘΜ
ΔήΗ«ΚΟΤΩ»ϊΘ§Ζ¥Η¥…œœ¬ΒΏΒΙΘ§“Γ‘»ΘΜ
Δί”Ο…ΌΝΩΒΡ’τΝσΥ°œ¥Β”…’±≠ΡΎ±ΎΚΆ≤ΘΝßΑτ2~3¥ΈΘ§œ¥Β”“ΚΉΣ“ΤΒΫ»ίΝΩΤΩ÷–ΘΜ
Θ®4Θ©‘Ύ Β―ι÷–ΤδΥϊ≤ΌΉςΨυ’ΐ»ΖΘ§»γ Β―ιΙΐ≥Χ÷–»±…Ό≤Ϋ÷ηΔίΘ§Μα Ι≈δ÷Τ≥ωΒΡNaOH»ή“Κ≈®Ε» Θ®ΧνΓΑΤΪΗΏΓ±ΜρΓΑΤΪΒΆΓ±ΜρΓΑ≤Μ±δΓ±Θ©ΘΜ»τΕ®»ί ±―ω ”ΩΧΕ»œΏΘ§‘ρΥυΒΟ»ή“Κ≈®Ε» 2.0 molΓΛL-1Θ®ΧνΓΑ¥σ”ΎΓ±ΜρΓΑ–Γ”ΎΓ±ΜρΓΑΒ»”ΎΓ±Θ©ΓΘ
Θ®1Θ©C Θ®2Θ©8.0 Θ®3Θ©ΔΌΔέΔίΔΎΔή Θ®4Θ©ΤΪΒΆ –Γ”Ύ
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚΘ®1Θ©≈δ÷Τ2.0 molΓΛL-1NaOH»ή“Κ90mLΘ§–η“Σ…’±≠ΓΔ100ml»ίΝΩΤΩΓΔΫΚΆΖΒΈΙήΓΔ≤ΘΝß±≠Θ§≤Μ–η“Σ¬©ΕΖΓΘ
Θ®2Θ©«ΓΒ±ΒΡ»ίΝΩΤΩΈΣ100mlΘ§Υυ“‘m(NaOH)=0.1LΓΝ2.0mol/LΓΝ40g/mol=8.0gΓΘ
Θ®3Θ©Α¥’’≥ΤΝΩ-»ήΫβ-ά以-œ¥Β”-Ε®»ί-“Γ‘»Θ§Ϋχ––≈≈–ρΘΚΔΌΔέΔίΔΎΔήΓΘ
Θ®4Θ©»±…Ό≤Ϋ÷ηΔίΘ§»ή÷ Φθ…ΌΘ§≈δ÷Τ»ή“Κ≈®Ε»ΤΪΒΆΘΜΕ®»ί ±―ω ”ΩΧΕ»œΏΘ§»ή“ΚΧεΜΐΤΪ¥σΘ§≈®Ε»ΤΪΒΆΘ§–Γ”Ύ2.0 molΓΛL-1ΓΘ
ΩΦΒψΘΚ±ΨΧβΩΦ≤ι≈δ÷Τ“ΜΕ®Έο÷ ΒΡΝΩ≈®Ε»ΒΡ»ή“ΚΓΘ

 100Ζ÷¥≥ΙΊΤΎΡ©≥ε¥ΧœΒΝ–¥πΑΗ
100Ζ÷¥≥ΙΊΤΎΡ©≥ε¥ΧœΒΝ–¥πΑΗ