ΧβΡΩΡΎ»ί
Θ®15Ζ÷Θ©ΧΰA «“Μ÷÷÷Ί“ΣΒΡΜυ±ΨΜ·ΙΛ‘≠ΝœΘ§”Ο÷ ΤΉΖ®≤βΒΟΤδœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ28ΓΘœ¬ΆΦ «“‘AΈΣ‘≠ΝœΚœ≥…“©Έο÷–ΦδΧεEΚΆ ς÷§KΒΡ¬ΖœΏΓΘ
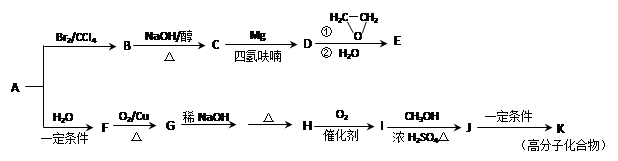
“―÷ΣΘΚI.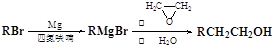
II.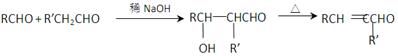
Θ®RΓΔRΓ·±μ ΨΧΰΜυΜρ«β‘≠Ή”Θ©
Θ®1Θ©A÷–ΙΌΡήΆ≈ΒΡΫαΙΙΦρ Ϋ « ΓΘ”–ΜζΈοBΒΡΟϊ≥Τ
Θ®2Θ©BΓζCΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘBΚΆ«β―θΜ·ΡΤΒΡΥ°»ή“ΚΦ”»»Ζ¥”ΠΥυΒΟΒΫΒΡ”–Μζ≤ζΈοΚΆ““ΕΰΥαΖ¥”Π…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοΘ§–¥≥ω…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ
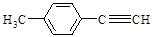
Θ®3Θ©EΒΡΖ÷Ή” ΫΈΣC4H8OΓΘœ¬Ν–ΙΊ”ΎEΒΡΥΒΖ®’ΐ»ΖΒΡ « Θ®ΧνΉ÷ΡΗ–ρΚ≈Θ©ΓΘ
a. Ρή”κΫπ τΡΤΖ¥”Π b. Ζ÷Ή”÷–4ΗωΧΦ‘≠Ή”“ΜΕ®Ι≤ΤΫΟφ
c. “ΜΕ®ΧθΦΰœ¬Θ§Ρή”κ≈®«βδεΥαΖ¥”Π d. ”κCH2=CHCH2OCH2CH3ΜΞΈΣΆ§œΒΈο
Θ®4Θ©GΓζH…φΦΑΒΫΒΡΖ¥”Πάύ–Ά”– ΓΘ
Θ®5Θ©IΒΡΖ÷Ή” ΫΈΣC4H6O2Θ§ΤδΫαΙΙΦρ ΫΈΣ ΓΘ
Θ®6Θ©JΓζKΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
Θ®7Θ©–¥≥ω”κEΨΏ”–œύΆ§ΙΌΡήΆ≈ΒΡΥυ”–Ά§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΘΚ
Θ®≤ΜΩΦ¬«Υ≥Ζ¥“λΙΙΘ§≤ΜΩΦ¬«ΓΣOHΝ§‘ΎΥΪΦϋΧΦ…œΒΡΫαΙΙΘ©ΓΘ

“―÷ΣΘΚI.
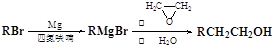
II.

Θ®RΓΔRΓ·±μ ΨΧΰΜυΜρ«β‘≠Ή”Θ©
Θ®1Θ©A÷–ΙΌΡήΆ≈ΒΡΫαΙΙΦρ Ϋ « ΓΘ”–ΜζΈοBΒΡΟϊ≥Τ
Θ®2Θ©BΓζCΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘBΚΆ«β―θΜ·ΡΤΒΡΥ°»ή“ΚΦ”»»Ζ¥”ΠΥυΒΟΒΫΒΡ”–Μζ≤ζΈοΚΆ““ΕΰΥαΖ¥”Π…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοΘ§–¥≥ω…ζ≥…ΗΏΖ÷Ή”Μ·ΚœΈοΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ
Θ®3Θ©EΒΡΖ÷Ή” ΫΈΣC4H8OΓΘœ¬Ν–ΙΊ”ΎEΒΡΥΒΖ®’ΐ»ΖΒΡ « Θ®ΧνΉ÷ΡΗ–ρΚ≈Θ©ΓΘ
a. Ρή”κΫπ τΡΤΖ¥”Π b. Ζ÷Ή”÷–4ΗωΧΦ‘≠Ή”“ΜΕ®Ι≤ΤΫΟφ
c. “ΜΕ®ΧθΦΰœ¬Θ§Ρή”κ≈®«βδεΥαΖ¥”Π d. ”κCH2=CHCH2OCH2CH3ΜΞΈΣΆ§œΒΈο
Θ®4Θ©GΓζH…φΦΑΒΫΒΡΖ¥”Πάύ–Ά”– ΓΘ
Θ®5Θ©IΒΡΖ÷Ή” ΫΈΣC4H6O2Θ§ΤδΫαΙΙΦρ ΫΈΣ ΓΘ
Θ®6Θ©JΓζKΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΓΘ
Θ®7Θ©–¥≥ω”κEΨΏ”–œύΆ§ΙΌΡήΆ≈ΒΡΥυ”–Ά§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΘΚ
Θ®≤ΜΩΦ¬«Υ≥Ζ¥“λΙΙΘ§≤ΜΩΦ¬«ΓΣOHΝ§‘ΎΥΪΦϋΧΦ…œΒΡΫαΙΙΘ©ΓΘ
Θ®1Θ©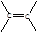 1,2-Εΰδε““Άι
1,2-Εΰδε““Άι
Θ®2Θ©BrCH2CH2Br + NaOH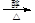 CH2="CHBr" + NaBr + H2O
CH2="CHBr" + NaBr + H2O
nHOOCCOOH+nHOCH2CH2OH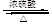
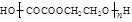 +(2nΘ≠1)H2O
+(2nΘ≠1)H2O
Θ®3Θ©ac
Θ®4Θ©Φ”≥…Ζ¥”ΠΓΔœϊ»ΞΖ¥”Π
Θ®5Θ©CH3CH=CHCOOH
Θ®6Θ©n CH3CH=CHCOOCH3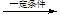
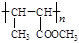
Θ®7Θ©CH3CH=CHCH2OH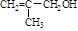
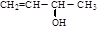
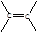 1,2-Εΰδε““Άι
1,2-Εΰδε““ΆιΘ®2Θ©BrCH2CH2Br + NaOH
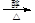 CH2="CHBr" + NaBr + H2O
CH2="CHBr" + NaBr + H2O nHOOCCOOH+nHOCH2CH2OH
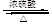
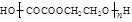 +(2nΘ≠1)H2O
+(2nΘ≠1)H2O Θ®3Θ©ac
Θ®4Θ©Φ”≥…Ζ¥”ΠΓΔœϊ»ΞΖ¥”Π
Θ®5Θ©CH3CH=CHCOOH
Θ®6Θ©n CH3CH=CHCOOCH3
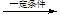
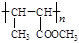
Θ®7Θ©CH3CH=CHCH2OH
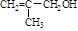
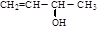
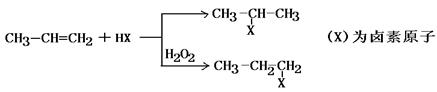
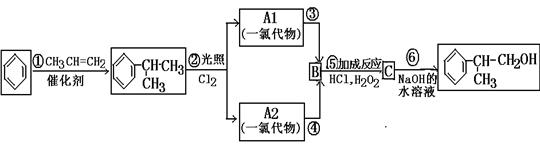
‘ΧβΖ÷ΈωΘΚœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ28ΒΡΧΰ÷ΜΩ…ΡήΈΣC2H4Θ§AΈΣCH2=CH2Θ§‘ρBΈΣCH2BrCH2BrΘ§BΓζCΒΡΖ¥”ΠΈΣœϊ»ΞΖ¥”ΠΓΘ”…–≈œΔIΦΑCΓζDΓζEΖ¥”ΠΧθΦΰΩ…÷ΣBΓζCœϊ»Ξ“ΜΖ÷Ή”HBrΘ§CΈΣCH2=CHBrΘ§DΈΣCH2=CHMgBrΘ§EΈΣCH2=CHCH2CH2OHΓΘ
CH2=CH2”κH2OΖΔ…ζΦ”≥…Ζ¥”Π…ζ≥…CH3CH2OHΘ®FΘ©Θ§GΈΣCH3CHOΘ§ΡΘΖ¬–≈œΔIIΩ…ΆΤ÷ΣHΈΣCH3CH=CHCHOΘ§IΈΣCH3CH=CHCOOHΘ§JΈΣCH3CH=CHCOOCH3Θ§KΈΣ
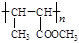 ΓΘ
ΓΘΘ®1Θ©““œ©÷–ΙΌΡήΆ≈ΈΣΧΦΧΦΥΪΦϋΘ§ΫαΙΙΦρ ΫΈΣ
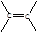 Θ§BΒΡΟϊ≥ΤΈΣ1Θ§2-Εΰδε““ΆιΓΘ
Θ§BΒΡΟϊ≥ΤΈΣ1Θ§2-Εΰδε““ΆιΓΘΘ®2Θ©B…ζ≥…CΈΣœϊ»ΞΖ¥”ΠΘ§ΖΫ≥Χ ΫΈΣBrCH2CH2Br + NaOH
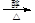 CH2="CHBr" + NaBr + H2OΓΘBrCH2CH2BrΚΆ«β―θΜ·ΡΤΥ°»ή“ΚΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…HOCH2CH2OHΘ§ΥϋΚΆ““ΕΰΥαΡήΖΔ…ζΥθΨέΖ¥”ΠΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣnHOOCCOOH+nHOCH2CH2OH
CH2="CHBr" + NaBr + H2OΓΘBrCH2CH2BrΚΆ«β―θΜ·ΡΤΥ°»ή“ΚΖΔ…ζΥ°ΫβΖ¥”Π…ζ≥…HOCH2CH2OHΘ§ΥϋΚΆ““ΕΰΥαΡήΖΔ…ζΥθΨέΖ¥”ΠΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣnHOOCCOOH+nHOCH2CH2OH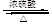
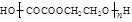 +(2nΘ≠1)H2OΓΘ
+(2nΘ≠1)H2OΓΘΘ®3Θ©EΈΣCH2=CHCH2CH2OHΘ§Ζ÷Ή”÷–Κ§”–τ«ΜυΘ§Ρή”κNa…ζ≥…«βΤχΘ§a’ΐ»ΖΘΜCH2=CHCH2Θ≠÷–3ΗωΧΦ‘≠Ή”“ΜΕ®Ι≤ΤΫΟφΘ§Θ≠CH2OH÷–ΧΦ‘≠Ή”Ω…ΡήΙ≤ΟφΘ§“≤Ω…Ρή≤ΜΙ≤ΟφΘ§b¥μΈσΘΜCH2=CHCH2CH2OHΡή”κ«βδεΥαΖ¥”Π…ζ≥…CH2=CHCH2CH2BrΚΆH2OΘ§c’ΐ»ΖΘΜΆ§œΒΈο÷ΗΫαΙΙœύΥΤΘ§Ζ÷Ή”Ήι≥…œύ≤νnΗωCH2ΒΡΜ·ΚœΈοΘ§CH2=CHCH2CH2OH τ”Ύ¥ΦάύΘ§CH2=CHCH2OCH2CH3 τ”ΎΟ―άύΘ§Εΰ’ΏΜΞΈΣΆ§Ζ÷“λΙΙΧεΘ§d¥μΈσΓΘ
Θ®4Θ©GΓζH…φΦΑΒΡΖ¥”ΠΈΣœ»Φ”≥…Κσœϊ»ΞΓΘ
Θ®5Θ©IΈΣCH3CH=CHCOOHΓΘ
Θ®6Θ©JΓζKΈΣΦ”ΨέΖ¥”ΠΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣn CH3CH=CHCOOCH3
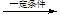
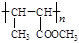 ΓΘ
ΓΘΘ®7Θ©EΈΣCH2=CHCH2CH2OHΘ§”κEΨΏ”–œύΆ§ΙΌΡήΆ≈ΒΡΆ§Ζ÷“λΙΙΧεΜΙ”–3÷÷Θ§ΫαΙΙΦρ ΫΈΣΘΚ
CH3CH=CHCH2OH ΓΔ
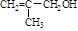 ΓΔ
ΓΔ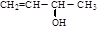 ΓΘ
ΓΘΒψΤάΘΚ±ΨΧβΤπ Φ‘≠ΝœΦρΒΞΘ§÷–ΦδΙΐ≥ΧΆΤΕœ ±“ΣΫαΚœΧβΗχ–≈œΔΘ§Ζώ‘ρΩ…ΡήΜα ΙΥΦΈ§ ήΉηΓΘ

ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ
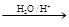 RΘ≠CH2CHO + RΓδOH
RΘ≠CH2CHO + RΓδOH 
 Θ©ΒΡ“ΜΧθ¬ΖœΏ»γœ¬ΘΚ
Θ©ΒΡ“ΜΧθ¬ΖœΏ»γœ¬ΘΚ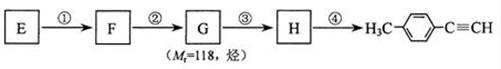 Θ®4Θ©–¥≥ωGΒΡΫαΙΙΦρ ΫΘΚ____________________________________ΓΘ
Θ®4Θ©–¥≥ωGΒΡΫαΙΙΦρ ΫΘΚ____________________________________ΓΘ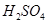 , Γς
, Γς
 ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ
ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ΜΞΈΣΆ§Ζ÷“λΙΙΧεΒΡΖΦœψΉεΜ·ΚœΈο”– ÷÷Θ§Τδ÷–“Μ÷÷Ά§Ζ÷“λΙΙΧεΒΡΚΥ¥≈Ι≤’ώ«βΤΉ”–»ΐ÷÷άύ–Ά«β‘≠Ή”ΒΡΈϋ ’ΖεΘ§ΗΟΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΈΣ ΓΘ
ΜΞΈΣΆ§Ζ÷“λΙΙΧεΒΡΖΦœψΉεΜ·ΚœΈο”– ÷÷Θ§Τδ÷–“Μ÷÷Ά§Ζ÷“λΙΙΧεΒΡΚΥ¥≈Ι≤’ώ«βΤΉ”–»ΐ÷÷άύ–Ά«β‘≠Ή”ΒΡΈϋ ’ΖεΘ§ΗΟΆ§Ζ÷“λΙΙΧεΒΡΫαΙΙΦρ ΫΈΣ ΓΘ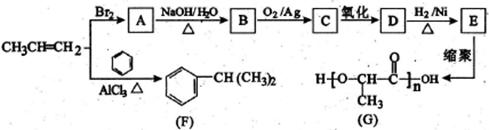


 CH3COOC2H5
CH3COOC2H5
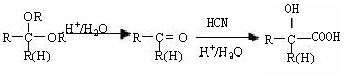
 Θ§XΩ…ΡήΖΔ…ζ
Θ§XΩ…ΡήΖΔ…ζ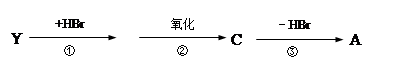
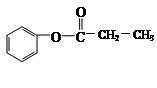 ΓΔ__________________________ΓΔ
ΓΔ__________________________ΓΔ