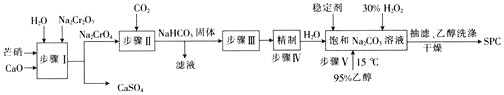
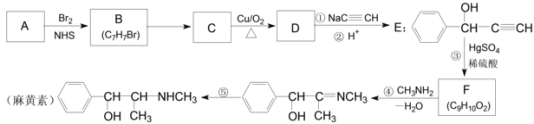
ЬтФПФкШн
ЁОЬтФПЁПЙЄвЕЩЯгУФбШмгкЫЎЕФЬМЫсяШ(SrCO3)ЗлФЉЮЊдСЯ(КЌЩйСПБЕКЭЬњЕФЛЏКЯЮя)жЦБИИпДПСљЫЎТШЛЏяШОЇЬх(SrCl26H2O)ЃЌЦфЙ§ГЬЮЊЃК
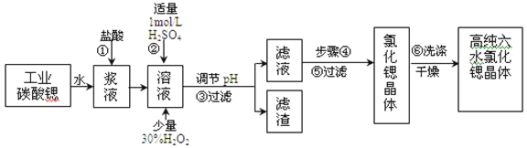
вбжЊЃКI.гаЙиЧтбѕЛЏЮяГСЕэЕФpHЃК
ЧтбѕЛЏЮя | Fe(OH)3 | Fe(OH)2 |
ПЊЪМГСЕэЕФpH | 1.5 | 6.5 |
ГСЕэЭъШЋЕФpH | 3.7 | 9.7 |
II.SrCl26H2OОЇЬхдк61ЁцЪБПЊЪМЪЇШЅНсОЇЫЎЃЌ100ЁцЪБЪЇШЅШЋВПНсОЇЫЎЁЃ
ЧыЛиД№ЃК
ЂХдкВНжшЂкжаМгШыЩйСПЕФ30% H2O2ЃЌЗДгІЕФРызгЗНГЬЪН_______________ЁЃ
ЂЦдкВНжшЂлжаЃЌашвЊНЋШмвКЕФpHгЩ1ЕїНкжС3.7вдЩЯЃЌЪЪвЫгУбЁЕФЪдМСЮЊ__________ЁЃЙ§ТЫЫљЕУТЫдќЕФжївЊГЩЗжЪЧ_______________ЁЃ
ЂЧЙигкЩЯЪіСїГЬжаЕФВНжшЂмЁЂЂнЁЂЂоЕФЫЕЗЈЃЌе§ШЗЕФЪЧ____________ЁЃ
A. ВНжшЂмАќРЈгУ60ЁцЕФШШЫЎдЁМгШШеєЗЂЕНШмвКБэУцГіЯжОЇФЄЁЂРфШДНсОЇ
B. ПЩвдЭЈЙ§НЕЕЭНсОЇЫйТЪЕФЗНЗЈРДЕУЕННЯДѓПХСЃЕФSrCl26H2OОЇЬх
C. ФГШмвКНЕЮТКѓШєЮоОЇЬхЮіГіЃЌПЩгУВЃСЇАєНСЖЏЛђЧсЧсФІВСШнЦїБк
D. ВНжшЂнЮЊГУШШЙ§ТЫЃЌВНжшЂоЕФЯДЕгМСЮЊБЅКЭ![]() ШмвК
ШмвК
ЂШЙЄвЕЩЯВЩгУМѕбЙКцИЩЛђепгУ50ЁЋ60ЁцЕФШШЗчДЕИЩSrCl26H2OОЇЬхЕФдвђЪЧ______________ЁЃ
ЂЩЮЊСЫВтЖЈЫљЕУSrCl26H2OОЇЬхбљЦЗЕФДПЖШЃЌЩшМЦСЫШчЯТЗНАИЃКГЦШЁ1.40gбљЦЗШмНтгкЪЪСПЫЎжаЃЌЯђЦфжаМгШыКЌAgNO3 2.38gЕФЯѕЫсвјШмвК(ШмвКжаГ§ClЃЭтЃЌВЛКЌЦфЫќгыAg+ЗДгІЩњГЩГСЕэЕФРызг)ЃЌClЃМДБЛШЋВПГСЕэЁЃШЛКѓгУКЌFe3+ЕФШмвКзїжИЪОМСЃЌгУ0.2000 molЁЄLЃ1ЕФNH4SCNБъзМШмвКЕЮЖЈЪЃгрЕФAgNO3ЃЌЪЙЪЃгрЕФAg+вдAgSCNАзЩЋГСЕэЕФаЮЪНЮіГіЃЌвдВтЖЈSrCl26H2OОЇЬхбљЦЗЕФДПЖШЁЃгУШЅЩЯЪіХЈЖШЕФNH4SCNШмвК20.00mLЃЌдђдSrCl26H2OОЇЬхЕФДПЖШЮЊ_____________________ЁЃ
ЁОД№АИЁП2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2O SrOЁЂSrCO3ЛђSr(OH)2 Fe(OH)3ЁЂBaSO4 AB ЗРжЙSrCl26H2OОЇЬхдк61ЁцвдЩЯЪБЪЇШЅНсОЇЫЎ 95.3%
ЁОНтЮіЁП
вдSrCO3ЮЊдСЯжЦБИСљЫЎТШЛЏяШЃЈSrCl26H2OЃЉЃЌгЩСїГЬПЩжЊЃЌSrCO3КЭбЮЫсЗДгІКѓШмвКжаГ§КЌгаSr2+КЭCl-ЭтЃЌЛЙКЌгаЩйСПFe2+ЁЂBa2+дгжЪЃЌШЛКѓМгСђЫсЩњГЩСђЫсБЕГСЕэЃЌМгШыЙ§бѕЛЏЧтЃЌЕїНкШмвКpHПЩЩњГЩЧтбѕЛЏЬњГСЕэЃЌЫљвдЙ§ТЫКѓТЫдќЮЊСђЫсБЕКЭЧтбѕЛЏЬњЃЌТЫвКжаКЌSrCl2ЃЌзюКѓеєЗЂЁЂРфШДНсОЇЕУЕНSrCl26H2OЃЌвдДЫРДНтД№ЁЃ
ЂХдкВНжшЂкжаМгШыЩйСПЕФ30% H2O2ЕФФПЕФЪЧНЋFe2+бѕЛЏГЩFe3+ЃЌЗДгІЕФРызгЗНГЬЪНЮЊ2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2OЃЛ
ЙЪД№АИЮЊЃК2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2OЃЛ
ЂЦЕїНкШмвКЕФpHФПЕФЪЧЪЙЬњРызгГСЕэЃЛЕїНкШмвКЕФpHЕФЭЌЪБВЛФмв§ШыЦфЫћдгжЪЃЌЫљвдбЁдёSrOЁЂSrCO3ЛђSr(OH)2ЕШЃЛвђЮЊЬМЫсяШЙЬЬхжаЛЙКЌгаЩйСПБЕЕФЛЏКЯЮяЃЌЫљвдВйзїЂлжаЫљЕУТЫдќЕФжївЊГЩЗжЪЧFe(OH)3ЁЂBaSO4ЃЛ
ЙЪД№АИЮЊЃКSrOЁЂSrCO3ЛђSr(OH)2ЕШЃЛFe(OH)3ЁЂBaSO4ЃЛ
ЂЧВНжшЂмАќРЈеєЗЂХЈЫѕЁЂРфШДНсОЇЃЌЮЊЗРжЙЮТЖШЙ§ИпЪЙгУSrCl26H2O ОЇЬхЪЇЫЎЃЌвђДЫВЩгУ60ЁцЕФШШЫЎдЁМгШШеєЗЂЕНШмвКБэУцГіЯжОЇФЄЃЌШЛКѓРфШДНсОЇЃЌAе§ШЗЃЛНсОЇЫйТЪдНТ§ЃЌЕУЕНЕФОЇЬхПХСЃдНДѓЃЌВЩгУНЕЕЭНсОЇЫйТЪЕФЗНЗЈРДЕУЕННЯДѓПХСЃЕФSrCl26H2O ОЇЬхЃЌBе§ШЗЃЛжЛгаШмвКДяЕНЙ§БЅКЭКѓШдЮоОЇЬхЮіГіЃЌВХПЩгУВЃСЇАєНСЖЏЛђЧсЧсФІВСШнЦїБкЕФЗНЗЈЪЙОЇЬхЮіГіЃЌCДэЮѓЃЛВНжшЂмЪЧРфШДНсОЇЃЌвђДЫВНжшЂнЪЧГЃЮТЙ§ТЫЛђМѕбЙЙ§ТЫЃЌВНжшЂоКѓЕУЕНИпДПSrCl26H2O ОЇЬхЃЌЫљвдбЁдёЕФЮоЛњЯДЕгМСЮЊБЅКЭТШЛЏяШШмвКЃЌМѕаЁОЇЬхЕФШмНтЭЌЪБвВВЛЛсВњЩњЦфЫћдгжЪЃЌDДэЮѓЃЛ
ЙЪД№АИЮЊЃКAЁЂBЃЛ
ЂШЙЄвЕЩЯВЩгУМѕбЙКцИЩЛђепгУ50ЁЋ60ЁцЕФШШЗчДЕИЩSrCl26H2OОЇЬхЪЧЮЊСЫЗРжЙSrCl26H2OОЇЬхдк61ЁцвдЩЯЪБЪЇШЅНсОЇЫЎЃЛ
ЙЪД№АИЮЊЃКЗРжЙSrCl26H2OОЇЬхдк61ЁцвдЩЯЪБЪЇШЅНсОЇЫЎЃЛ
ЂЩn(NH4SCN) = 0.2000 molЁЄLЃ1ЁС0.02L =0.004molЃЌЮДЗДгІЕФn(Ag+) = n(NH4SCN) = 0.004molЃЌдђn(ClЃ)= ![]() ЃЌдђдSrCl26H2OОЇЬхЕФДПЖШЮЊЃК
ЃЌдђдSrCl26H2OОЇЬхЕФДПЖШЮЊЃК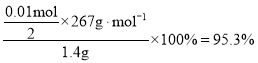 ЃЛ
ЃЛ
ЙЪД№АИЮЊЃК95.3%ЁЃ

ЁОЬтФПЁПвбжЊЃК![]() ЃЌРћгУЯТЭМзАжУгУе§ЖЁДМКЯГЩе§ЖЁШЉЁЃЯрЙиЪ§ОнШчЯТЃК
ЃЌРћгУЯТЭМзАжУгУе§ЖЁДМКЯГЩе§ЖЁШЉЁЃЯрЙиЪ§ОнШчЯТЃК

ЮяжЪ | ЗаЕу/Ёц | УмЖШ/ЃЈgЁЄ | ЫЎжаШмНтад |
е§ЖЁДМ | 117.2 | 0.8109 | ЮЂШм |
е§ЖЁШЉ | 75.7 | 0.8017 | ЮЂШм |
ЯТСаЫЕЗЈжаЃЌе§ШЗЕФЪЧ ЃЈ ЃЉ
A. ЯђЛёЕУЕФДже§ЖЁШЉжаМгШыЩйСПН№ЪєФЦЃЌМьбщЦфжаЪЧЗёКЌгае§ЖЁДМ
B. ЕБЮТЖШМЦ1ЪОЪ§ЮЊ90ЁЋ95ЁцЃЌЮТЖШМЦ2ЪОЪ§дк117.2ЁцзѓгвЪБЃЌЪеМЏВњЮя
C. ЗДгІНсЪјЃЌНЋСѓГіЮяЕЙШыЗжвКТЉЖЗжавдЗжШЅЫЎВуЃЌДже§ЖЁШЉДгЗжвКТЉЖЗЩЯПкЕЙГі
D. ЮЊМгПьЗДгІЃЌгІНЋЫсЛЏЕФNa2Cr2O7ШмвКвЛДЮадШЋВПМгШые§ЖЁДМжа