��Ŀ����
��ÿ��2�֣���18�֣���.��֪��
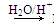 R��CH2CHO��R��OH����ϩ����A����Է�������(Mr)Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4����A��صķ�Ӧ���£�
R��CH2CHO��R��OH����ϩ����A����Է�������(Mr)Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4����A��صķ�Ӧ���£�
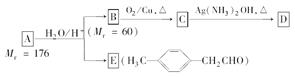
��ش��������⣺
(1)A�ķ���ʽΪ________��
(2)B��������________��
(3)д��C�D��D��Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
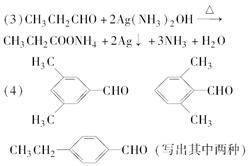
(4)д������ͬʱ��������������E��ͬ���칹��Ľṹ��ʽ��________��________��
�����ڷ���ȩ��
�ڱ����������ֲ�ͬ��������ԭ�ӡ�
��.��Eת��Ϊ�Լ�����Ȳ

(5)д���١��ܲ���Ӧ�����Լ�����Ӧ�����͢١��۲���Ӧ���ͣ�

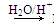 R��CH2CHO��R��OH����ϩ����A����Է�������(Mr)Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4����A��صķ�Ӧ���£�
R��CH2CHO��R��OH����ϩ����A����Է�������(Mr)Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4����A��صķ�Ӧ���£�
��ش��������⣺
(1)A�ķ���ʽΪ________��
(2)B��������________��
(3)д��C�D��D��Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(4)д������ͬʱ��������������E��ͬ���칹��Ľṹ��ʽ��________��________��
�����ڷ���ȩ��
�ڱ����������ֲ�ͬ��������ԭ�ӡ�
��.��Eת��Ϊ�Լ�����Ȳ

(5)д���١��ܲ���Ӧ�����Լ�����Ӧ�����͢١��۲���Ӧ���ͣ�
| ��� | �����Լ�����Ӧ���� | ��Ӧ���� |
| �� | | |
| �� | | |
| �� | | |
| �� | | |
(1)C12H16O��(2)1����(��������)
(5)

(5)
| ��� | �����Լ�����Ӧ���� |
| �� | H2������(��Ni��Pt��Pd)���� |
| �� | ŨH2SO4���� |
| �� | Br2(��Cl2) |
| �� | NaOH��C2H5OH���� |
���������(1)��ø���Ϊ�أ��ȸ���Ϊ��,��Ϊ�أ��٣���������12X+Y+16=176
����X=12 Y="16" ����A�ķ���ʽΪC12H16O��
��2��A�����Ե�ˮ��Һ�б��B��E��A��������ϩ�����ѣ��ܼӳɺ�ˮ�⡣E����9��C��A����12����˵��B����3��������ΪC����Ǿ���������Һ��˵����C����-CHO�����ţ���B��һ����������̼�Ĵ�����B����Է�������Ϊ60����BΪ������
��3��C
 D��������Ӧ����CΪ��ȩ���ʷ���ʽΪ
D��������Ӧ����CΪ��ȩ���ʷ���ʽΪCH3CH2CHO��2[Ag(NH3)2]OH
 CH3CH2COONH4��2Ag��3NH3��H2O
CH3CH2COONH4��2Ag��3NH3��H2O
��4��ͬ���칹�壺��ѧʽ��ͬ���ṹ��ͬ�Ļ����
��5���������֪E�Ļ�ѧʽΪ
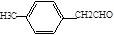 ,F�Ļ�ѧʽΪ
,F�Ļ�ѧʽΪ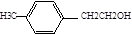 ,G�Ļ�ѧʽ��
,G�Ļ�ѧʽ��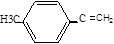 ��H�Ļ�ѧʽ��
��H�Ļ�ѧʽ��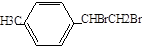 �����üӳɡ���ȥ��±������ȥ�ķ���������Ƴɼ���Ȳ��
�����üӳɡ���ȥ��±������ȥ�ķ���������Ƴɼ���Ȳ�����������⿼���ڶ෴Ӧ���ͣ������ӳɡ���ȥ��±���ȣ���Ϥ�����л�����ת����������Ч�Ľ�������ƶ��⣬���������е��Ѷȡ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
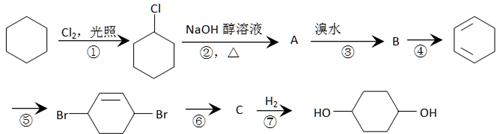
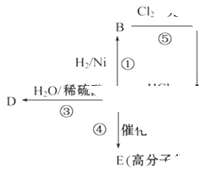

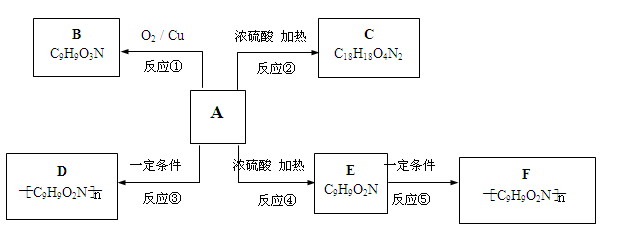
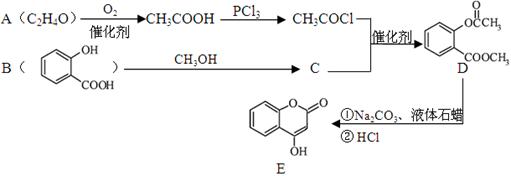

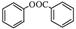 ����һ����Ҫ���л��ϳ��м��塣��д���Ա����ױ�Ϊԭ����ȡ�û�����ĺϳ�·������ͼ����ԭ����ѡ����
����һ����Ҫ���л��ϳ��м��塣��д���Ա����ױ�Ϊԭ����ȡ�û�����ĺϳ�·������ͼ����ԭ����ѡ����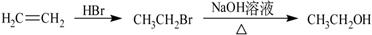
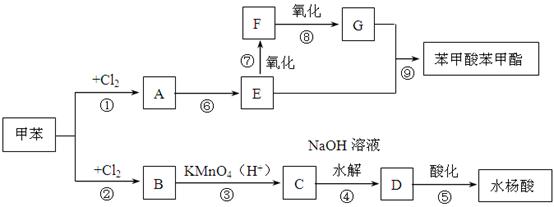
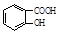 ����ش�
����ش�
 ��R1��R2����������
��R1��R2���������� ��C�ܷ���������Ӧ���ҷ�������֧����
��C�ܷ���������Ӧ���ҷ�������֧����