��Ŀ����
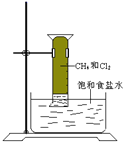
����Ŀ��ij��ȤС��̽��SO2���廹ԭFe3��������ʹ�õ�ҩƷ��װ������ͼ��ʾ������˵������������(����)
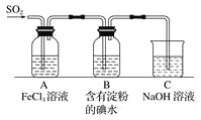
A.�ܱ���I���Ļ�ԭ������SO2��������B����ɫ��Һ��ɫ
B.װ��C������������SO2β������ֹ��Ⱦ����
C.Ϊ����֤A�з�����������ԭ��Ӧ������KMnO4��Һ���Ϻ�ɫ��ȥ
D.Ϊ����֤A�з�����������ԭ��Ӧ��������ϡ�����ữ��BaCl2��Һ��������ɫ����
���𰸡�C
��������
A. ���е��۵ĵ�ˮ��ͨ��SO2���壬�ᷢ����Ӧ��SO2��I2��2H2O===H2SO4��2HI������I2��Ӧ�����ģ����B����ɫ��Һ��ɫ��֤�����ʵĻ�ԭ�ԣ�SO2>I������A��ȷ��
B. SO2�Ǵ�����Ⱦ�����SO2���������壬������NaOH������Ӧ��SO2��2NaOH=Na2SO3��H2O�����Կ�����NaOH��Һ����β������ֹ��Ⱦ��������B��ȷ��
C. ��SO2��FeCl3��������Ӧ����A�м���KMnO4��Һ��������Ӧ��2KMnO4��5SO2�� 2H2O=K2SO4��2MnSO4��2H2SO4����Һ�Ϻ�ɫ��ȥ����SO2��FeCl3������Ӧ��SO2��2H2O��2FeCl3=H2SO4��2HCl��2FeCl2���������Һ�м������Ը��������Һʱ���ᷢ����Ӧ��MnO4-��5Fe2����8H+=Mn2���� 5Fe3����4H2O����Һ�Ϻ�ɫҲ��ȥ����˲�����֤A���Ƿ�����������ԭ��Ӧ����C����
D. ��A�з�����������ԭ��Ӧ��SO2��2H2O��2FeCl3=H2SO4��2HCl��2FeCl2����Һ�к������ᣬ��������ϡ�����ữ��BaCl2��Һʱ���ᷢ����Ӧ��H2SO4�� BaCl2=BaSO4����2HCl��������ɫ��������û�з���������ԭ��Ӧ������������HCl>H2SO3��������Һ�м�����ϡ�����ữ��BaCl2��Һʱ����������ɫ��������D��ȷ��
��ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50mL0.50mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL0.55mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У���Ͼ��ȣ���û��Һ����¶ȡ�
�ش��������⣺
(1)д���÷�Ӧ���Ȼ�ѧ����ʽ����֪����lmolҺ̬ˮ�ķ�Ӧ��Ϊ��57.3kJ/mol��______________________��
(2)�ֽ�һ������ϡ����������Һ��ϡ����������Һ��ϡ��ˮ�ֱ�� 1L1mol/L����ǡ����ȫ��Ӧ���䷴Ӧ�ȷֱ�ΪH1��H2��H3����H1��H2��H3�Ĵ�С��ϵΪ________________________��
(3)�������������������Һ���ܶȶ���1g/cm3����֪�кͷ�Ӧ��������Һ�ı�����c=4.18J/(g����)��Ϊ�˼����к��ȣ�ijѧ��ʵ���¼���������
ʵ����� | ��ʼ�¶� | ��ֹ�¶� | |
���� | ����������Һ | �����Һ | |
1 | 20.0 | 20.2 | 23.2 |
2 | 20.2 | 20.4 | 23.4 |
3 | 20.4 | 20.6 | 23.6 |
4 | 20.1 | 20.3 | 26.9 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к���H_____(�������һλС��)��
(4)�����60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������__________ (���ȡ�����ȡ�)�������к���__________(���ȡ�����ȡ�)��
(5)���ü������ȼƲ�������������������������Һ�кͷ�Ӧ�ķ�Ӧ�ȣ����д�ʩ�������ʵ�龫�ȵ�����_______��
A��������Һ��(��ȷ��0.01 mL)������Ͳ(��ȷ��0.1 mL)��ȡ��ӦҺ
B�����ٽ�����Һ��ϣ����ٽ��貢��¼����¶�
C�����ڡ���Ͳ֮�����������ʣ���ֹ������ʧ
D��������Ϊ500����¶ȼƴ�������Ϊ100����¶�