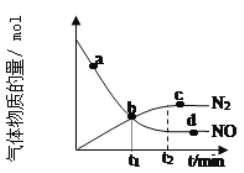
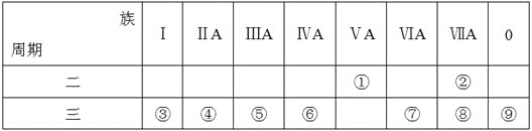
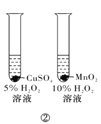
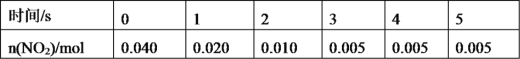��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣���Ԫ�ط��Ż�ѧʽ��ɸ�С�⡣
IA | ���� | IIIA | IVA | VA | VIA | VIIA | 0 | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
4 | �� | �� | �� |
(1)��ѧ��������õ�Ԫ��__________________���ǽ�������ǿ��Ԫ����___________________�����ȶ�����̬�⻯����___________________������������Ӧˮ����������ǿ����_____________________________________________��
(2)��������ǿ�ĵ�����ˮ��Ӧ�����ӷ���ʽΪ______________��
(3)�ۢߢ������Ԫ�صļ����Ӱ뾶�ɴ�С��˳��Ϊ_________________��
(4)�ֱ�д���ޡ��ߺ����γɵ���̬�⻯��Ľṹʽ___________________��
(5)�õ���ʽ��ʾ�ڵ���������γɹ���____________________________��
���𰸡�Ar F HF HClO4 2K+2H2O=2OH-+2K++H2�� r(Cl-)��r(N3-)��r(F-)��r(Mg2+) ![]() ��
��![]()
![]()
![]()
![]()
��������
����Ԫ�������ڱ��е�λ��֪���١���Ԫ�طֱ���Na��K��Mg��Ca��Al��C��O��F��Cl��Br��ArԪ�أ��ݴ˽��
(1)ϡ�����廯ѧ��������ã��⼸��Ԫ���У���ѧ��������õ���ArԪ�أ��ǽ�������ǿ��Ԫ��λ�����ڱ����Ͻ�(ϡ���������)�������⼸��Ԫ���зǽ�������ǿ����FԪ�أ����ȶ�����̬�⻯��ΪHF����O��F�����ۣ�������������Ӧˮ����������ǿ����HClO4��
(2)��������ǿ�ĵ�����K��K��ˮ��Ӧ����KOH��������������Ӧ�����ӷ���ʽΪ2K+2H2O=2OH-+2K++H2����
(3)Cl-��O2-��F-��Mg2+��һ�����Ӳ㣬��O2-��F-��Mg2+�����ӽṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶�ɴ�С��˳��Ϊr(Cl-)��r(N3-)��r(F-)��r(Mg2+)��
(4)C��O��H�γɵļ��⻯��ΪCH4��H2O�����ǵĽṹʽ����Ϊ![]() ��
��![]() ��
��
(5)K��������ΪK2O�������ӻ�����õ���ʽ��ʾ���γɹ���Ϊ![]()
![]()
![]() ��
��

 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�