��Ŀ����
����Ŀ��298Kʱ����0.01molL-1NaOH��Һ�ζ�10mL0.01molL-1H2A��Һ�ĵζ�������ͼ��ʾ����֪��25��ʱ��H2A��Ka1=10-1.75��Ka2=10-7.19��������˵������ȷ���ǣ� ��
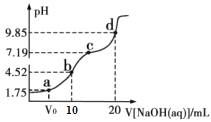
A.a��������Һ�У�V0=5mL
B.B��������Һ�У�c(H2A)��c(H��)= c(A2-)��c(OH-)
C.C��������Һ�У�c(Na��)>3 c(HA-)
D.D��������Һ��A2-ˮ��ƽ�ⳣ��Kh1=10-6.81
���𰸡�A
��������
A��a��������Һ�У�pH=1.75����c(H2A)=![]() = c(HA-)������H2A��NaOH��Ӧ������c(H2A)>c(HA-)��V0<5mL��A����ȷ��
= c(HA-)������H2A��NaOH��Ӧ������c(H2A)>c(HA-)��V0<5mL��A����ȷ��
B��B��ʱ��H2A��KOH�պ���ȫ��Ӧ����KHA����Һ�д�������ƽ����ϵ��HA-![]() H++A2-��HA-+H2O
H++A2-��HA-+H2O![]() H2A+OH-��H2O
H2A+OH-��H2O![]() H++OH-���ɴ˵ó�������Һ�У�c(H2A)��c(H��)= c(A2-)��c(OH-)��B��ȷ��
H++OH-���ɴ˵ó�������Һ�У�c(H2A)��c(H��)= c(A2-)��c(OH-)��B��ȷ��
C��C��ʱ��c(HA-)=![]() = c(A2-)�����ݵ���غ�c(Na��)+c(H+)= c(HA-)+2 c(A2-)+ c(OH-)=3c(HA-)+ c(OH-)����ΪpH=7.19��c(H+)< c(OH-)������c(Na��)>3 c(HA-)��C��ȷ��
= c(A2-)�����ݵ���غ�c(Na��)+c(H+)= c(HA-)+2 c(A2-)+ c(OH-)=3c(HA-)+ c(OH-)����ΪpH=7.19��c(H+)< c(OH-)������c(Na��)>3 c(HA-)��C��ȷ��
D��D��������Һ�У�A2-ˮ��ƽ�ⳣ��Kh1= ![]() =
=![]() =10-6.81��D��ȷ��
=10-6.81��D��ȷ��
��ѡA��

����Ŀ��һ������Na2CO3��NaHCO3�ľ��Ȼ����ֳ��������ȵ����ݣ��ֱ���50mL��ͬŨ�ȵ����ᷴӦ���õ��������������������������ϵ���±���ʾ��
�顡�� | 1 | 2 | 3 |
��������/g | 3.80 | 6.20 | 7.20 |
��������/g | 1.76 | 2.64 | 2.64 |
(1)�ϱ���_______�����ݱ�������μӷ�Ӧ����ʣ�࣬������______________��
(2)�ϱ��е�2������������____________��������������������������������������
(3)ͨ��������Na2CO3��NaHCO3������������Ϊ_________��____________��