题目内容
已知铜在常温下能被稀硝酸溶解,其反应的化学方程式如下
3Cu + 8HNO3 =3Cu(NO3)2 + 2NO↑+ 4H2O
(1)用双线桥法分析上述反应(只需标出电子得失的方向和数目)
(2)上述反应中氧化剂是 ,氧化产物是 ;
;
(3)硝酸在反应过程中起的作用是 、 ;
硝酸没有全部参加氧化还原反应,没有参加氧化还原反应的硝酸占总硝酸的 ;
(4)若反应中转移了0.6mol电子,产生的气体在标准状况下的体积是
。
3Cu + 8HNO3 =3Cu(NO3)2 + 2NO↑+ 4H2O
(1)用双线桥法分析上述反应(只需标出电子得失的方向和数目)
(2)上述反应中氧化剂是 ,氧化产物是
 ;
;(3)硝酸在反应过程中起的作用是 、 ;
硝酸没有全部参加氧化还原反应,没有参加氧化还原反应的硝酸占总硝酸的 ;
(4)若反应中转移了0.6mol电子,产生的气体在标准状况下的体积是
。
(1)
失去3*2e-
│ ̄ ̄ ̄ ̄ ̄ ̄ ̄ ̄↓
3Cu+8HNO3(稀)=3Cu(NO3)2+4H2O+2NO↑
│_______ _______________↑
_______________↑
得2*3e-,
(2) 稀硝酸 , 硝酸铜 ;(2分)
(3) 氧化剂 、 酸 ;(各1 分) 3/4 ;(2分)
分) 3/4 ;(2分)
(4) 4.48L 。(2分)
失去3*2e-
│ ̄ ̄ ̄ ̄ ̄ ̄ ̄ ̄↓
3Cu+8HNO3(稀)=3Cu(NO3)2+4H2O+2NO↑
│_______
 _______________↑
_______________↑得2*3e-,
(2) 稀硝酸 , 硝酸铜 ;(2分)
(3) 氧化剂 、 酸 ;(各1
 分) 3/4 ;(2分)
分) 3/4 ;(2分)(4) 4.48L 。(2分)
略

练习册系列答案
 高效智能课时作业系列答案
高效智能课时作业系列答案 捷径训练检测卷系列答案
捷径训练检测卷系列答案
相关题目


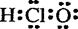
 2H+ + S2-
2H+ + S2-