��Ŀ����
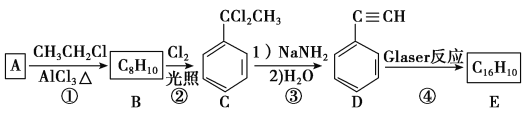
����Ŀ����֪A��B��C�ǽ������ʣ��ס��ҡ���Ϊ�������壬����B�ǵؿ��к����ӵڶ�λ�Ľ���Ԫ�ء�����֮���ܷ������·�Ӧ��ͼ����Щ��Ӧ�IJ���ͷ�Ӧ������û��ȫ���������

�����������Ϣ�ش��������⣺
��1��д���������ʵĻ�ѧʽ�� B____________�������_______________��
��2��д����Ӧ������ѧ����ʽ��__________________________________��
д����Ӧ������ѧ����ʽ��_________________________��
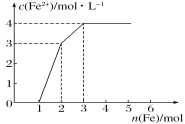
��3������F����Һ��ͨ������Ŀ����Ͱ����Ļ�����壬ͬ�����������ɫ����H��д��������Ӧ���ܵ����ӷ���ʽ________________�����˺�������Һ�������ӵķ���Ϊ______________��
���𰸡���1��Fe��1������HCl��1������
��2��2FeCl2+Cl2=2FeCl3��2������2FeCl3+Cu=CuCl2+2FeCl2��2������
��3��4Fe2+ + O2 + 8NH3 + 10H2O = 8NH4+ + 4Fe(OH)3����2������ȡ��Һ�������Թ��У�����ŨNaOH��Һ�����������Թ�����ʪ�����ɫʯ����ֽ����ų������壬����ֽ�����������NH4+����2�֣�
��������
�����������֪A��B��C�ǽ������ʣ�A����ɫ��Ӧ�Ի�ɫ��A��Na����ˮ��Ӧ�����������ƺ�������������������D���������ơ�����ɫ�������������������Ȼ��⣬����ˮ�õ����ᡣB�ǵؿ��к����ӵڶ�λ�Ľ���Ԫ����B�����������ᷴӦ�����Ȼ�������������F���Ȼ���������������Ӧ�����Ȼ�����G���Ȼ�������ɫ����C��ͭ�����Ȼ�����Ӧ�����Ȼ��������Ȼ�ͭ���Ȼ������������Ʒ�Ӧ���ɺ��ɫ��������������
��1��B������������ѧʽ�ֱ���Fe��HCl��
��2����Ӧ������ѧ����ʽΪ2FeCl2+Cl2=2FeCl3����Ӧ������ѧ����ʽΪ2FeCl3+Cu=CuCl2+2FeCl2��
��3������F����Һ��ͨ������Ŀ����Ͱ����Ļ�����壬ͬ�����������ɫ����H��������Ӧ���ܵ����ӷ���ʽΪ4Fe2+ + O2 + 8NH3 + 10H2O = 8NH4+ + 4Fe(OH)3�������˺�������Һ��笠����ӵķ���Ϊȡ��Һ�������Թ��У�����ŨNaOH��Һ�����������Թ�����ʪ�����ɫʯ����ֽ����ų������壬����ֽ�����������NH4+��