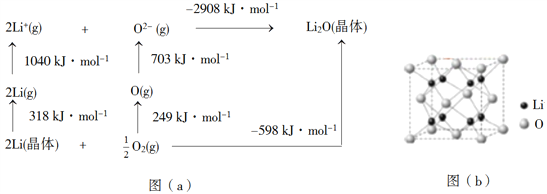
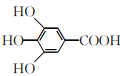
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΥς œ÷ΤΦν”÷≥ΤΑ±ΦνΖ®Θ§Τδ÷ς“Σ…ζ≤ζΝς≥Χ»γΉσœ¬ΆΦ:
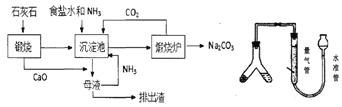
(1)¥÷―Έ÷–≥ΘΚ§Ca2+ΓΔMg2+ΓΔSO42-Β»‘”÷ άκΉ”Θ§Ω…“ά¥ΈΦ”»κNaOHΓΔ____ΓΔ____ΓΔ―ΈΥα ‘ΦΝά¥Ϋχ––Χα¥ΩΓΘ
(2)”ΟΜ·―ßΖΫ≥Χ Ϋ±μ Ψ≥ΝΒμ≥Ί÷–ΖΔ…ζΒΡΖ¥”ΠΈΣ________________________________ΓΘ
(3)Α±ΦνΖ®÷ΤΒΟΒΡ¥ΩΦν―υΤΖ÷–Ω…ΡήΜαΚ§”–MaCl‘”÷ ΓΘœ÷”ΟΝΩΤχΖ®≤βΕ®¥ΩΦν―υΤΖΒΡ¥ΩΕ»Θ§(»γ”“…œΆΦ)»γΚΈΦλ≤ιΗΟΉΑ÷ΟΒΡΤχΟή–‘_________________________________Θ§≥Τ»ΓmΩΥ―υΤΖΉΑ»κY–ΆΙήΉσ≤ύΘ§ΝΩΤχΙή÷–“ΚΧε”ΠΈΣ______________(ΧνΉ÷ΡΗ)ΓΘ
A.Υ° B.±ΞΚΆ ≥―ΈΥ° C.±ΞΚΆΧΦΥαΡΤ»ή“Κ D.±ΞΚΆΧΦΥα«βΡΤ»ή“Κ
(4)»τΉνΚσ≤βΒΟ≤ζ…ζCO2ΤχΧεΒΡΧεΜΐΈΣVL(±ξΩωœ¬)Θ§‘ρ―υΤΖ÷–¥ΩΦνΒΡ¥ΩΕ»ΈΣ_________ΓΘ»τΉνΚσΕΝ ΐ ±Θ§ΖΔœ÷Υ°ΉΦΙή“ΚΟφΗΏ”ΎΝΩΤχΙή(ΤδΥϊ≤ΌΉςΕΦ’ΐ»Ζ)Θ§‘ρΦΤΥψΥυΒΟ¥ΩΦν―υΤΖΒΡ¥ΩΕ»÷Β_____(ΧνΓΑΤΪ¥σΓ±Θ§ΓΑΤΪ–ΓΓ±ΜρΓΑΈό”ΑœλΓ±)ΓΘ
ΓΨ¥πΑΗΓΩBaCl2Na2CO3NaClΘΪNH3+CO2+H2O=NaHCO3ΓΐΘΪNH4Cl‘ΎΥ°ΉΦΙή÷–Φ”Υ°Θ§ ΙΥ°ΉΦΙή“ΚΟφΗΏ”ΎΝΩΤχΙήΘ§“ΜΕΈ ±ΦδΚσ“ΚΟφΗΏΕ»±Θ≥÷≤Μ±δD106V/22.4mΤΪ–Γ
ΓΨΫβΈωΓΩ
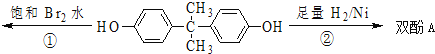
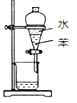
±ΨΧβΩΦ≤ιΜ·―ßΙΛ“’Νς≥ΧΚΆΜ·―ß Β―ιΖΫΑΗΒΡ…ηΦΤΓΘ
”…Νς≥ΧΆΦΩ…÷ΣΘ§ΧΦΥαΗΤλ―…’…ζ≥…―θΜ·ΗΤΚΆΕΰ―θΜ·ΧΦΘ§Εΰ―θΜ·ΧΦΆ®»κΖ¥”Π≥Ί÷–”ꬻ̷ΡΤ»ή“ΚΚΆΑ±ΤχΖ¥”Π…ζ≥…ΧΦΥα«βΡΤ≥ΝΒμΚΆ¬»Μ·οß»ή“ΚΘΜΧΦΥα«βΡΤΖ÷Ϋβ…ζ≥…ΧΦΥαΡΤΚΆΕΰ―θΜ·ΧΦΘ§…ζ≥…ΒΡΕΰ―θΜ·ΧΦΆ®»κΖ¥”Π≥Ί―≠ΜΖ Ι”ΟΘΜ―θΜ·ΗԔꬻ̷οßΖ¥”Π…ζ≥…¬»Μ·ΗΤ≈≈≥ΐ‘ϋΚΆΑ±ΤχΘ§Α±ΤχΆ®»κΖ¥”Π≥Ί―≠ΜΖ Ι”ΟΓΘ
ΫβΈωΘΚΘ®1Θ©Φ”»κ«β―θΜ·ΡΤ»ή“Κά¥≥ΐ»Ξ¥÷―Έ÷–ΒΡΟΨάκΉ”Θ§Φ”»κ¬»Μ·±Β»ή“Κ≥ΐ»ΞSO42-άκΉ”Θ§Φ”Na2CO3»ή“ΚΦ»Ω…“‘≥ΐ»Ξ»ή“Κ÷–ΒΡΗΤάκΉ”ΜΙΩ…“‘≥ω»Ξ»ή“Κ÷–ΙΐΝΩΒΡ±ΒάκΉ”Θ§Ιΐ¬ΥΚσΦ”»κ―ΈΥαΩ…“‘≥ΐ»ΞΙΐΝΩΒΡΧΦΥαΡΤΚΆ«β―θΜ·ΡΤΘΜΘ®2Θ©¬»Μ·ΡΤ»ή“ΚΓΔΑ±ΤχΚΆΕΰ―θΜ·ΧΦ‘Ύ≥ΝΒμ≥Ί÷–Ζ¥”Π…ζ≥…ΧΦΥα«βΡΤΚΆ¬»Μ·οßΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚNaCl+NH3+CO2+H2O®TNaHCO3Γΐ+NH4ClΘΜΘ®3Θ©Φλ≤ιΝΩΤχΉΑ÷ΟΒΡΤχΟή–‘Θ§Ω…¥”Υ°ΉΦΙήΦ”»κΥ°÷Ν”κΝΩΤχΙή–Έ≥…“ΚΟφ≤νΘ§»γ“ΜΕΈ ±Φδ“ΚΟφ≤Μ±δΜ·Θ§ΥΒΟςΤχΟή–‘ΝΦΚΟΘ§Ζώ‘ρ¬©ΤχΘΜ“ρΈΣΖ¥”Π…ζ≥…ΒΡΕΰ―θΜ·ΧΦ»ή”ΎΥ°Θ§”Ο±ΞΚΆΧΦΥα«βΡΤ»ή“ΚΩ…“‘ΫΒΒΆΕΰ―θΜ·ΧΦΒΡ»ήΫβΕ»Θ§Φθ–Γ Β―ιΈσ≤νΘ§Ι ―ΓDΘΜΘ®4Θ©»τΉνΚσ≤βΒΟ≤ζ…ζCO2ΤχΧεΒΡΧεΜΐΈΣVL(±ξΩωœ¬)Θ§”…ΧΦ‘≠Ή”Ηω ΐ ΊΚψΩ…÷ΣΘ§nΘ®Na2CO3Θ©= nΘ®CO2Θ©=![]() molΘ§mΩΥ―υΤΖ÷–ΧΦΥαΡΤΒΡ÷ ΝΩΈΣ
molΘ§mΩΥ―υΤΖ÷–ΧΦΥαΡΤΒΡ÷ ΝΩΈΣ![]() ΓΝ106gΘ§‘ρ―υΤΖ÷–¥ΩΦνΒΡ¥ΩΕ»ΈΣ
ΓΝ106gΘ§‘ρ―υΤΖ÷–¥ΩΦνΒΡ¥ΩΕ»ΈΣ![]() ΓΝ100%ΘΜ»τΉνΚσΕΝ ΐ ±Θ§ΖΔœ÷Υ°ΉΦΙή“ΚΟφΗΏ”ΎΝΩΤχΙήΘ§ΥΒΟςΝΩ»ΓΒΡΕΰ―θΜ·ΧΦΤχΧεΧεΜΐΤΪ–ΓΘ§‘ρΦΤΥψΥυΒΟ¥ΩΦν―υΤΖΒΡ¥ΩΕ»÷ΒΤΪ–ΓΓΘ
ΓΝ100%ΘΜ»τΉνΚσΕΝ ΐ ±Θ§ΖΔœ÷Υ°ΉΦΙή“ΚΟφΗΏ”ΎΝΩΤχΙήΘ§ΥΒΟςΝΩ»ΓΒΡΕΰ―θΜ·ΧΦΤχΧεΧεΜΐΤΪ–ΓΘ§‘ρΦΤΥψΥυΒΟ¥ΩΦν―υΤΖΒΡ¥ΩΕ»÷ΒΤΪ–ΓΓΘ

 ÷–ΩΦάϊΫΘ÷–ΩΦ ‘ΨμΜψ±ύœΒΝ–¥πΑΗ
÷–ΩΦάϊΫΘ÷–ΩΦ ‘ΨμΜψ±ύœΒΝ–¥πΑΗΓΨΧβΡΩΓΩΔώ.»ΥΧε―Σ“ΚάοCa2ΘΪΒΡ≈®Ε»“ΜΑψ≤…”Οmg/cm3ά¥±μ ΨΓΘ≥ι»Γ“ΜΕ®ΧεΜΐΒΡ―Σ―υΘ§Φ” ΝΩΒΡ≤ίΥαοß[(NH4)2C2O4]»ή“ΚΘ§Ω…Έω≥ω≤ίΥαΗΤ(CaC2O4)≥ΝΒμΘ§ΫΪ¥Υ≤ίΥαΗΤ≥ΝΒμœ¥Β”Κσ»ή”Ύ«ΩΥαΩ…ΒΟ≤ίΥα(H2C2O4)Θ§‘Ό”ΟKMnO4»ή“ΚΒΈΕ®Φ¥Ω…≤βΕ®―Σ“Κ―υΤΖ÷–Ca2ΘΪΒΡ≈®Ε»ΓΘΡ≥―–ΨΩ–‘―ßœΑ–ΓΉι…ηΦΤ»γœ¬ Β―ι≤Ϋ÷η≤βΕ®―Σ“Κ―υΤΖ÷–Ca2ΘΪΒΡ≈®Ε»ΓΘ
Θ®≈δ÷ΤKMnO4±ξΉΦ»ή“ΚΘ©
»γΆΦΥυ Ψ «≈δ÷Τ50 mL KMnO4±ξΉΦ»ή“ΚΒΡΙΐ≥Χ Ψ“βΆΦΓΘ

(1)«κΡψΙέ≤λΆΦ Ψ≈–ΕœΘ§Τδ÷–≤Μ’ΐ»ΖΒΡ≤ΌΉς”–__________ (Χν–ρΚ≈)ΓΘ
(2)Τδ÷–»ΖΕ®50 mL»ή“ΚΧεΜΐΒΡ»ίΤς «________(ΧνΟϊ≥Τ)ΓΘ
(3)»γΙϊ”ΟΆΦ ΨΒΡ≤ΌΉς≈δ÷Τ»ή“ΚΘ§Υυ≈δ÷ΤΒΡ»ή“Κ≈®Ε»ΫΪ________(ΧνΓΑΤΪ¥σΓ±ΜρΓΑΤΪ–ΓΓ±)ΓΘ
Θ®≤βΕ®―Σ“Κ―υΤΖ÷–Ca2ΘΪΒΡ≈®Ε»Θ©
≥ι»Γ―Σ―υ20.00 mLΘ§Ψ≠Ιΐ…œ ω¥ΠάμΚσΒΟΒΫ≤ίΥαΘ§‘Ό”Ο0.020 molΓΛLΘ≠1 KMnO4»ή“ΚΒΈΕ®Θ§ Ι≤ίΥαΉΣΜ·≥…CO2“ί≥ωΘ§’β ±Ι≤œϊΚΡ12.00 mL KMnO4»ή“ΚΓΘ
(4)≈δΤΫ≤ίΥα”κKMnO4Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ__MnOΘΪ H2C2O4ΘΪ HΘΪ=== Mn2ΘΪΘΪ CO2ΓϋΘΪ H2OΓΘ
(5)ΒΈΕ®÷’Βψ ±ΒΡœ÷œσ «_____________________________________
(6)Ψ≠ΙΐΦΤΥψΘ§―Σ“Κ―υΤΖ÷–Ca2ΘΪΒΡ≈®Ε»ΈΣ__________m molΓΛcmΘ≠3ΓΘ
Δρ. Ρ≥–ΓΉιΆ§―ß…ηΦΤ»γœ¬ Β―ιΘ§―–ΨΩ―«Χζ―Έ”κH2O2»ή“ΚΒΡΖ¥”ΠΓΘ
Β―ι1 ‘ΦΝΘΚΥαΜ·ΒΡ0.5 molΓΛLΘ≠1FeSO4»ή“Κ(pH ΘΫ 0.2)Θ§5% H2O2»ή“Κ(pH ΘΫ 5)ΓΘ
≤ΌΉς | œ÷œσ |
»Γ2 mL…œ ωFeSO4»ή“Κ”Ύ ‘Ιή÷–Θ§Φ”»κ5ΒΈ5% H2O2»ή“Κ | »ή“ΚΝΔΦ¥±δΈΣΉΊΜΤ…ΪΘ§…‘ΚσΘ§≤ζ…ζΤχ≈ίΓΘ≤βΒΟΖ¥”ΠΚσ»ή“ΚpHΘΫ0.9 |
œρΖ¥”ΠΚσΒΡ»ή“Κ÷–Φ”»κKSCN»ή“Κ | »ή“Κ±δΚλ |
(1)H2O2ΒΡΒγΉ” Ϋ «_______Θ§…œ ω Β―ι÷–H2O2»ή“Κ”κFeSO4»ή“ΚΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «_________ΓΘ
(2)≤ζ…ζΤχ≈ίΒΡ‘≠“ρ «____________________________________________ΓΘ
ΓΨΧβΡΩΓΩœ¬Ν– Β―ιΉΑ÷ΟΜρ≤ΌΉς’ΐ»Ζ«“Ρή¥οΒΫ Β―ιΡΩΒΡΒΡ «
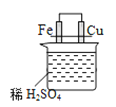
AΘ°÷Τ±Η«β―θΜ·―«Χζ | BΘ° ·”ΆΒΡ’τΝσ | CΘ°Ζ÷άκ±ΫΚΆΥ° | DΘ°±»ΫœFeΓΔCuΒΡΫπ τΜνΕ·–‘ |
|
|
|
|
A. AB. BC. CD. D