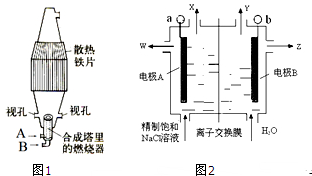
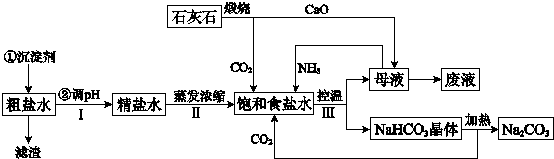
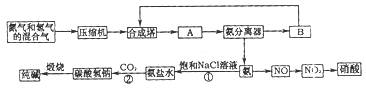
��Ŀ����
������һ��ȡ��Ʒ�ܽ���Լ�ʹCO32-�������ⶨ������������
������������ϡ�ὫCO32-ת��ΪCO2���ⶨCO2��������
��1������һ�IJ��������У��ٳ�����ƷW1 g���ܽ���Ʒ���ڼ���������BaCl2��Һ���۹��ˣ���ϴ�ӣ��ݸ����������W2 g�����к��ز���������������W3 g��
����ʱ���趨��ʵ������Ϊ
| 106W3 |
| 197W1 |
| 106W3 |
| 197W1 |
��2����������ʵ��װ����ͼ��

���������У��ټ��װ�õ������ԣ����ڸ������װ����ʯ�ң���������ΪW1 g���۳���W2 g��Ʒװ����ƿB�У��ܻ���������������ӣ��ݹر�ֹˮ�У���������ϡH2SO4�����ٲ�������Ϊֹ����ֹˮ�У�����������������ӣ��ٳ�������ܣ�����ΪW3 g��
���ݷ����������ݣ������Ʒ�д������������Ϊ
| 53(W3-W1) |
| 22W2 |
| 53(W3-W1) |
| 22W2 |
��2������̼���������ᷴӦ���ɶ�����̼�����ʵ����������̼���Ƶ����ʵ������ټ����̼���Ƶ����������������к��ж�����̼�����������������ܱ���Һ���գ�Ũ���������ˮ�ԣ��ݴ˷��������û��װ��A��ʵ������ܢߣ��ⶨ�Ķ�����̼������ƫС���ⶨ���ƫ�ͣ������е�ˮ������������ܵ������¸�����������ӣ��ⶨ�����ɶ�����̼������ƫ���ƫ���ڸ���ܺ��һװ�м�ʯ�ҵĸ���ܣ����տ����е�ˮ�Ͷ�����̼�����Լ�С�ⶨ�����
| W3 |
| 197 |
| ||
| W1 |
| 106W3 |
| 197W1 |
�ʴ�Ϊ��������ƽ��
| 106W3 |
| 197W1 |
��2������ʯ�����յ������Ƕ�����̼��������̼�������ǣ�W3-W1��g������̼ԭ���غ��̼���Ƶ�����=
| 106(W3-W1) |
| 44 |
| ||
| W2 |
| 53(W3-W1) |
| 22W2 |
�����к��ж�����̼��A������������Һ�������տ����ж�����̼����ֹ����̼���ƵIJⶨ����Ӧ���ɵĶ�����̼�к���ˮ��������ʯ��������ˮ����������Ӱ�������̼�IJⶨ��������Cװ���е�Ũ��������ˮ���������û��װ��A��ʵ������ܢߣ��ᵼ�¸���������ɶ�����̼��������С���ⶨ���ƫ�ͣ������е�ˮ�����������ܶ�Ӱ�������̼�IJⶨ�����¶�����̼������ƫ�ⶨ���ƫ�ͣ�Ϊ��ֹˮ�����ĸ��ţ�Ӧ���ڸ�����ұ��ټ�һ��װ�м�ʯ�ҵĸ���ܣ�
�ʴ�Ϊ��
| 53(W3-W1) |
| 22W2 |

| A����������Ũ���ᡢŨ�����Ũ�����зֱ�Ͷ����������������������ܽ���� | B�����ȼҵ�У������ռ����������õ���������̬��������ںϳ����� | C�����������������ͨ����������������ƻ��ʺ�ըҩ�� | D����ҵ�����ҵ���ᶼ���ʻ�ɫ��������������ȫ��ͬ |
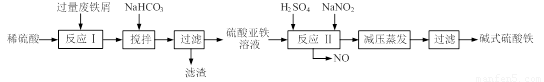
��12�֣���ʽ������[Fe(OH)SO4]��һ��������ˮ����������Ч����������ҽҩ��Ҳ���������������������Ѫ����ҵ�����÷���м�����������������������ȣ�������ʽ�������Ĺ����������£�
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
(1)��ҵ�ϳ���һ��Ũ�ȵĴ�����Һ��ϴ��м��Ŀ���� ��
(2)��������NaHCO3��Ŀ���ǵ�����Һ��pH�� a��a�ķ�Χ�� ��
(3)��֪�����£�Al(OH)3��Ksp=1.3��10-33����pH=5ʱ����Һ��c(Al3+)=
(4)��ʵ�������У���Ӧ��ͬʱͨ��O2�Լ���NaNO2�������������뷴Ӧ��O2��11.2L����״���������൱�ڽ�ԼNaNO2�����ʵ���Ϊ ��O2���뷴Ӧ�����ӷ���ʽΪ
(5)��ʽ����������ˮ�������Fe(OH)2+���ӣ��ɲ���ˮ������Fe2(OH)42+�ۺ����ӡ���ˮ�ⷴӦ�����ӷ���ʽΪ ��
��12�֣���ʽ������[Fe(OH)SO4]��һ��������ˮ����������Ч����������ҽҩ��Ҳ���������������������Ѫ����ҵ�����÷���м�����������������������ȣ�������ʽ�������Ĺ����������£�
��֪������������������������ʽ����ʱ��Һ��pH���±���
|
������ |
Fe(OH)3 |
Fe(OH)2 |
Al(OH)3 |
|
��ʼ���� |
2.3 |
7.5 |
3.4 |
|
��ȫ���� |
3.2 |
9.7 |
4.4 |
�ش��������⣺
(1)��ҵ�ϳ���һ��Ũ�ȵĴ�����Һ��ϴ��м��Ŀ���� ��
(2)��������NaHCO3��Ŀ���ǵ�����Һ��pH�� a��a�ķ�Χ�� ��
(3)��֪�����£�Al(OH)3��Ksp=1.3��10-33����pH=5ʱ����Һ��c(Al3+)=
(4)��ʵ�������У���Ӧ��ͬʱͨ��O2�Լ���NaNO2�������������뷴Ӧ��O2��11.2L����״���������൱�ڽ�ԼNaNO2�����ʵ���Ϊ ��O2���뷴Ӧ�����ӷ���ʽΪ
(5)��ʽ����������ˮ�������Fe(OH)2+���ӣ��ɲ���ˮ������Fe2(OH)42+�ۺ����ӡ���ˮ�ⷴӦ�����ӷ���ʽΪ ��