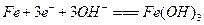��Ŀ����
��ʾ���з�Ӧ�����ӷ���ʽ��д����ȷ����
H++SO42��+Ba2++HCO3��==BaSO4��+CO2��+H2O
| A����Na2CO3��Һ�еμӼ���ϡ����: CO32��+H+==HCO3�� |
| B��ʯ������NH4HCO3Ũ��Һ��ϼ���: Ca(OH)2+NH4++HCO3��="=" CaCO3��+NH3��+2H2O |
| C��������Һ��������Ba(OH)2��Һ��Ӧ: Al3++2SO42��+2Ba2++4OH��==AlO2��+2BaSO4��+2H2O |
| D��������Ba(HCO3)2��Һ��NaHSO4��Һ���: |
B
ѡ��A������㣬CO32�D��H���������HCO3�D����ȷ��ѡ��B�����Ӽ�������������ѡ��C��Ba(OH)2��Һ��������ʹ������Һ�е�Al3��ת��ΪAlO2�D��SO42�D����BaSO4��������ȷ��ѡ��D����Ba(HCO3)2��Һ��������ʹ�����NaHSO4��Һ�ṩ��H����SO42�D��ȫ��Ӧ����CO2��BaSO4��H2O����ȷ��

��ϰ��ϵ�д�
�����Ŀ

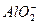 +2H2O
+2H2O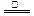 NH3����SO32����2H2O
NH3����SO32����2H2O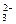 ��H2O
��H2O
 +2H
+2H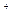
 H
H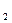 O����ȷ�����ӷ���ʽ��___________��
O����ȷ�����ӷ���ʽ��___________��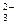 +2H
+2H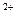 +Al
+Al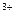 ����ȷ�����ӷ���ʽ��________��
����ȷ�����ӷ���ʽ��________��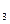 ������ȷ�����ӷ���ʽ��__________________________________________________________________��
������ȷ�����ӷ���ʽ��__________________________________________________________________��
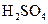 ��
��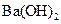 ��Һ��Ӧ��
��Һ��Ӧ��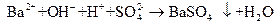

 ��Һ����
��Һ���� ���壺
���壺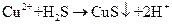
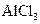 ��Һ�м��������Ũ��ˮ��
��Һ�м��������Ũ��ˮ��
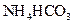 ϡ��Һ��ϣ�
ϡ��Һ��ϣ�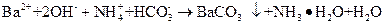
 H2��+ Cl2��
H2��+ Cl2��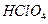 �ĵ��뷽��ʽ��
�ĵ��뷽��ʽ��