��Ŀ����
����Ŀ������п����������п���ס�ӡȾýȾ���ȡ���п����Ҫ�ɷ�ΪZnO��������FeO��CuO��SiO2�����ʣ��Ʊ�ZnSO4��7H2O���������¡�
��֪��Ksp[Fe(OH)3]��4.0��1038��Ksp[Cu(OH)2]��2.2��1020

��1��������1������Ҫ�ɷ���_______���ѧʽ����������������У�Ϊ�����пԪ�ؽ������ʣ��ɲ�ȡ�Ĵ�ʩ�У����ʵ�������Ũ�ȣ���_______����һ������
��2����������������������������ԭ��Ӧ�����ӷ���ʽ��_______��
��3���������������У�����ZnO����Fe(OH)3������ԭ����_______��
��4����������ZnO���壬��ֻ����Fe(OH)3������δ����Cu(OH)2�������Ҳ�ó��������Һ��c(Fe3��)��4.0��1014mol/L����ʱ��Һ��c(Cu2��)��ȡֵ��Χ��_______mol/L��
��5����������п�۵�������_______��
��6������Һ�õ�ZnSO47H2O�IJ�������Ϊ_______��_______�����ˡ�ϴ�ӡ����ʵ�����й��˲�����Ҫʹ�õIJ����������ձ���_______��_______��
���𰸡� SiO2 ����п����ֽ��转�ϻ��ʵ����ȵ� 2Fe2++H2O2+2H+=2Fe3++2H2O Fe3+����Һ�д���ˮ��ƽ��:Fe3++3H2O![]() Fe(OH)3+3H+,����ZnO����H+��Ӧ��H+Ũ�ȼ�С��ˮ��ƽ��������У�����Fe(OH)3���� <2.2��10-4 ��ȥ��Һ�е�Cu2+ ����Ũ�� ��ȴ�ᾧ ©�� ������
Fe(OH)3+3H+,����ZnO����H+��Ӧ��H+Ũ�ȼ�С��ˮ��ƽ��������У�����Fe(OH)3���� <2.2��10-4 ��ȥ��Һ�е�Cu2+ ����Ũ�� ��ȴ�ᾧ ©�� ������
��������������Ҫ������п���Ʊ�ZnSO4��7H2O�����̵����ۡ�
��1��������1������Ҫ�ɷ��Dz�����ϡ�����SiO2��������������У�Ϊ�����пԪ�ؽ������ʣ��ɲ�ȡ�Ĵ�ʩ�У����ʵ�������Ũ�ȣ��ڷ���п����ֽ��转�ϻ��ʵ����ȵȡ�
��2���������������У�����������ԭ��Ӧ�����ӷ���ʽ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��3���������������У�����ZnO����Fe(OH)3������ԭ����Fe3+����Һ�д���ˮ��ƽ��:Fe3++3H2O![]() Fe(OH)3+3H+������ZnO����H+��Ӧ��H+Ũ�ȼ�С��ˮ��ƽ��������У�����Fe(OH)3������
Fe(OH)3+3H+������ZnO����H+��Ӧ��H+Ũ�ȼ�С��ˮ��ƽ��������У�����Fe(OH)3������
��4��c(OH��)��( =10-8mol/L����ʱ��Һ��c(Cu2��)=
=10-8mol/L����ʱ��Һ��c(Cu2��)=  <2.2��10-4 mol/L��
<2.2��10-4 mol/L��
��5����������п�۵������dz�ȥ��Һ�е�Cu2+��
��6������Һ�õ�ZnSO47H2O�IJ�������Ϊ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����ʵ�����й��˲�����Ҫʹ�õIJ����������ձ���©������������

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ﹤ҵ��
����Cr(��)�Ĵ��������������£�

��֪���������ȡҺ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
�������£�����������������������ʽ����ʱ��Һ��pH���£�
������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
������ȫʱ��pH | 3.7 | 11.1 | 5.4(>8�ܽ�) | 9(>9�ܽ�) |
(1)ʵ������18.4 mol��L-1��Ũ��������480 mL 2 mol��L��1�����ᣬ����ȡŨ����___mL������ʱ���ò�����������Ͳ���ձ��Ͳ������⣬����____________________________��
(2)H2O2�������ǽ���Һ���е�Cr3+ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ��
___________________________________________��
(3)����NaOH��Һʹ��Һ�ʼ��ԣ��ȿ��Գ�ȥijЩ�������ӣ�ͬʱ�ֿ��Խ�Cr2O72-ת��Ϊ__________(�����Ļ�ѧʽ)
(4)�����ӽ�����֬�ķ�Ӧԭ��Ϊ��Mn+ + n NaR = MRn + n Na���������������ӽ�����֬�ɳ�ȥ��Һ���еĽ�����������__________________��
(5)д��������������SO2���л�ԭʱ������Ӧ�����ӷ���ʽ______________________________��
(6)�����ζ����Dzⶨ����Ũ�ȵķ���֮һ��Ϊ�˲ⶨij��ˮ��SCN��Ũ�ȣ����ñ�AgNO3��Һ�ζ�����Һ����֪��
�������� | AgCl | AgI | AgCN | Ag2CrO4 | AgSCN |
��ɫ | �� | �� | �� | ש�� | �� |
Ksp | 1.8��10-10 | 8.3��10-17 | 1.2��10-16 | 3.5��10-11 | 1.0��10-12 |
�ζ�ʱ��ѡΪ�ζ�ָʾ������____(ѡ����)���ζ��յ�������________________________��
A��NaCl B��K2CrO4 C��KI D��NaCN
����Ŀ����˾ƥ�֣�����ˮ������![]() ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128����135����ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ��������
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128����135����ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ��������

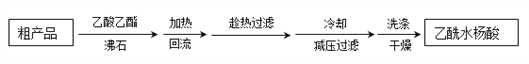
�Ʊ����������������£�
![]()
��Ҫ�Լ��Ͳ�Ʒ�������������±���ʾ��
���� | ��Է������� | �۵��е㣨���� | ˮ |
ˮ���� | 138 | 158���۵㣩 | �� |
������ | 102 | 139.4���е㣩 | ��ˮ�� |
����ˮ���� | 180 | 135���۵㣩 | �� |
�����������Ϣ�ش��������⣺
��1���Ʊ���˾ƥ��ʱ��Ҫʹ�ø����������ԭ����________________________________��
��2���ϳɰ�˾ƥ��ʱ��������ļ��ȷ�����______________________��

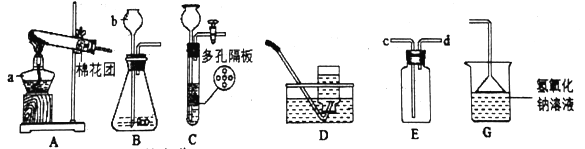
��3���ᴿ�ֲ�Ʒ�������£����Ȼ���װ����ͼ��
�ٷ�ʯ��������__________________________________��
������ˮ������������________________������b������c������
��ʹ���¶ȼƵ�Ŀ����_____________________________________________________��
��4����ʵ����ԭ��������2.0gˮ���ᡢ5.0mL��������![]() �������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ__________________���ðٷ�����ʾ��С�����һλ����
�������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ__________________���ðٷ�����ʾ��С�����һλ����