��Ŀ����
����Ŀ�����ױ����������Ȼ���������Ӧһ��ˮ��Ϲ��գ������л��Ȼ����յĸ���Ʒ�Ȼ���Ϊԭ���Ʊ�������ʵ���ȵ�ѭ�����ã�ԭ��Ϊ4HCl��g��+O2��g��![]() 2Cl2��g��+2H2O��g������HC1��O2�ֱ���5�����ʵ����ȣ���1��1 ��2��1 ��4��1 ��6��1 ��8��1��������ݻ��ɱ��������Ͷ��ʱ����Ӧ�¶ȶ�HClƽ��ת����Ӱ���������ͼ��
2Cl2��g��+2H2O��g������HC1��O2�ֱ���5�����ʵ����ȣ���1��1 ��2��1 ��4��1 ��6��1 ��8��1��������ݻ��ɱ��������Ͷ��ʱ����Ӧ�¶ȶ�HClƽ��ת����Ӱ���������ͼ��

��ش��������⣺
��1�������жϸ÷�Ӧ�Ѿ��ﵽ��ѧƽ�����__�� ������ĸ����
A���ܱ���������ѹǿ���� B���ܱ������л��������ܶȲ���
C��v��HCl��=2v��Cl2�� D���ܱ������������������������
��2��d���߶�Ӧ��Ͷ�ϱ���__�����ֵ���������ѡ����ͬ�����л���ҵ��Ҫ��O2���͵��������Ȼ��������壬�ɿ���n��HCl����n��O2��=_______�Ʊ���
��3���÷�Ӧ��ƽ�ⳣ������ʽΪ________��
��4������b���߶�Ӧ��Ͷ�ϱȽ��з�Ӧ�����¶�Ϊ415�棬��Ӧ�ﵽƽ��ʱCl2���������Ϊ_______��
���𰸡� AD 6:1 8:1 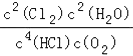 30.8%��
30.8%��
����������1��4HCl��g��+O2��g��2Cl2��g��+2H2O��g������Ӧ�����������С�ķ�Ӧ��
A���������ʵ��������仯�����ܱ���������ѹǿ���䣬˵����Ӧ�ﵽƽ��״̬����A��ȷ��B�������������䣬������䣬�ܱ������л��������ܶ�ʼ�ղ��䣬��B����C����Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮�ȣ�Ϊ����Ӧ����֮�ȣ�v��HCl��=2v��Cl2������˵�����淴Ӧ������ͬ������˵����Ӧ�ﵽƽ��״̬����C����D���ܱ������К������������������ƽ���־����D��ȷ����ѡAD��
��2����������������ʱ��O2����Խ��HCl��ת����Խ���ɴ˿�ȷ��aΪ1��1��bΪ2��1��cΪ4��1��dΪ6��1��eΪ8��1���л���ҵ��Ҫ��O2���͵��������Ȼ��������壬�ɿ���n��HCl����n��O2��=8��1����3���÷�Ӧ��ƽ�ⳣ������ʽΪ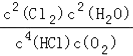 ����4����ͼ�ɶ���Ͷ�ϱ�Ϊ2��1���¶�Ϊ415��ʱ��HCl��ת����Ϊ80%����Ͷ���HClΪ2mol��O2Ϊ1mol���ɴ˿ɽ�������ʽ��
����4����ͼ�ɶ���Ͷ�ϱ�Ϊ2��1���¶�Ϊ415��ʱ��HCl��ת����Ϊ80%����Ͷ���HClΪ2mol��O2Ϊ1mol���ɴ˿ɽ�������ʽ��

����ƽ��������Cl2�����ʵ�������=![]() =30.8%��
=30.8%��

����Ŀ���屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�

�� | �� | �屽 | |
�ܶ�/(g��cm��3) | 0.88 | 3.10 | 1.50 |
�е�/�� | 80 | 59 | 156 |
ˮ���ܽ�� | �� | �� | �� |
�����кϳɲ���ش����⣺
(1)��a�м���15 mL��ˮ����������м����b��С�ļ���4.0 mLҺ̬�塣��a�е��뼸���壬�а�ɫ��������������Ϊ������ ���塣�����μ���Һ����ꡣװ��d�������� ��
(2)Һ�����������в�������ᴿ��
����a�м���10 mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10 mLˮ��8 mL 10%��NaOH��Һ��10 mLˮϴ�ӡ�NaOH��Һϴ�ӵ������� ��
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˡ������Ȼ��Ƶ�Ŀ���� ��
(3)�����Ϸ���������屽�л����е���Ҫ����Ϊ ��Ҫ��һ���ᴿ�����в����б������ (������ȷѡ��ǰ����ĸ)��
A���ؽᾧ B������ C������ D����ȡ
(4)�ڸ�ʵ���У�a���ݻ����ʺϵ��� (������ȷѡ��ǰ����ĸ)��
A��25 mL B��50 mL C��250 mL D��500 mL

