��Ŀ����
����Ŀ����.�������и����������������ݣ��ɷֱ��������Һ�ġ�������������������Һ�ġ����ʵ���Ũ�ȡ�������⡣
��1����֪ij����������ҺV L�к���N�����������ӣ����������Һ�����ʵ���Ũ����________��
��2����֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a������������Һ�����ʵ���������Ϊ________��
��.�����£�10.0 mL 1.0 mol��L��1��H2SO4(aq)����ˮϡ�͵�500 mL������H2SO4(aq)��Ũ��Ϊ________mol��L��1������Ũ����ʹ��Һ�����Ϊ2.40 mL������H2SO4(aq)��Ũ��Ϊ____mol��L��1��
��.��֪��״����1���ˮ���ܽ�500������Ȼ��⣬�õ��ܶ�Ϊ1.19g/mL�����ᣬ��������״�����Ȼ��ⱥ����Һ�����ʵ���������Ϊ______���������Ũ��Ϊ_____�����������һλС������
���𰸡�![]()
![]() 0.0204.244.9%14.6mol/L
0.0204.244.9%14.6mol/L
��������
��.��1��N�����������ӵ����ʵ���Ϊ![]() ����������Ϊǿ����ʣ����������Ƶ����ʵ���Ϊ
����������Ϊǿ����ʣ����������Ƶ����ʵ���Ϊ![]() ���������������Һ�����ʵ���Ũ��Ϊ
���������������Һ�����ʵ���Ũ��Ϊ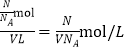 ��
��
��2����֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a��������������ˮ�����ʵ���֮��Ϊ1��a�����ߵ�����֮��Ϊ����40g/mol��1������18g/mol��a��=20��9a����˸���Һ���������Ƶ���������Ϊ![]() ��
��
��.�����£�10.0 mL 1.0 mol��L��1��H2SO4��aq������������ʵ���Ϊ��1.0mol/L��0.01L=0.01mol����ˮϡ�͵�500mL��ϡ���������ʵ����ʵ������䣬������������Һ��Ũ��Ϊ��0.01mol��0.5L=0.02mol/L������Ũ����������������ʵ������䣬����Ũ������Һ�����Ϊ2.40mL��������������Һ��Ũ��Ϊ0.01mol��0.0024L��4.2mol/L��
��.��֪��״����1���ˮ���ܽ�500������Ȼ��⣬�õ��ܶ�Ϊ1.19g/mL�����ᣬ�����ˮ��1L�����Ȼ�����500L����˱�״�����Ȼ��ⱥ����Һ�����ʵ���������Ϊ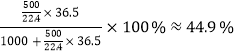 ������
������![]() ��֪�������Ũ��Ϊ
��֪�������Ũ��Ϊ![]() ��
��

 �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�