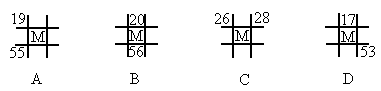��Ŀ����
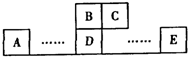 ��ͼΪԪ�����ڱ��е�һ���֣�A��B��C��D��E��Ϊ����������Ԫ�أ�BԪ��ԭ�ӵ��������4�����ӣ�Eԭ��������������C��ԭ��������ȣ��ش��������⣺
��ͼΪԪ�����ڱ��е�һ���֣�A��B��C��D��E��Ϊ����������Ԫ�أ�BԪ��ԭ�ӵ��������4�����ӣ�Eԭ��������������C��ԭ��������ȣ��ش��������⣺��1��B��D�γɵĻ�����ף�C��D�γɵĻ������ң����߾���ṹ��Ϊ�ռ���״�ṹ�����侧������Ϊ
ԭ�Ӿ���
ԭ�Ӿ���
���ҵĻ�ѧʽΪSi3N4
Si3N4
����2��B��D�ֱ����E�γɻ������������������
�Ǽ���
�Ǽ���
����Ǽ��ԡ����ԡ������ӣ��Խ��ס��ҡ��������������ʰ��۵��ɸߵ��͵�˳�����У�Si3N4��SiC��SiCl4��CCl4
Si3N4��SiC��SiCl4��CCl4
�����û�ѧʽ��ʾ����3��B��CԪ���γɵ����ʣ�BC��2�ɳ�Ϊ��±�أ�д����������NaOH��Һ��Ӧ�����ӷ���ʽ
��CN��2+2OH-=CN-+CNO-+H2O
��CN��2+2OH-=CN-+CNO-+H2O
����4��C��E�γɵķ��ӵĿռ乹��Ϊ�����Σ������и�ԭ����������8�����ȶ��ṹ���������ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ�����ɺ�C����ɫ���������塢C����ۺ����ἰE���⻯�д���÷�Ӧ�Ļ�ѧ����ʽ
3NCl3+5H2O=2NO+HNO3+9HCl
3NCl3+5H2O=2NO+HNO3+9HCl
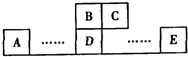
��������A��B��C��D��E��Ϊ����������Ԫ�أ��ɸ�Ԫ�ص����λ�ÿ�֪��B��C���ڵڶ����ڣ�A��D��E���ڵ������ڣ�BԪ��ԭ�ӵ��������4�����ӣ���BΪ̼Ԫ�أ���CΪ��Ԫ�أ�B��Dͬ���壬��DΪ��Ԫ�أ�Eԭ��������������C��ԭ��������ȣ���E����������Ϊ7����EΪ��Ԫ�أ�
��1���ס��ң�����ṹ��Ϊ�ռ���״�ṹ����������Ϊԭ�Ӿ��壻�ҵĻ�ѧʽΪSi3N4��
��2��ԭ�Ӿ���е���ڷ��Ӿ��壬�ף�SiC�����ң�Si3N4����ԭ�Ӿ��壬����C-Si����N-Si������N-Si�����ȶ���Si3N4�е�ߣ�����CCl4��������SiCl4�����Ƿ��Ӿ��壬�ṹ���ƣ���Է�������Խ�е�Խ�ߣ�
��3������������ˮ��Ӧ����HClO��HCl��д��
��4��NCl3�ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ������NO��HNO3��HCl��
��1���ס��ң�����ṹ��Ϊ�ռ���״�ṹ����������Ϊԭ�Ӿ��壻�ҵĻ�ѧʽΪSi3N4��
��2��ԭ�Ӿ���е���ڷ��Ӿ��壬�ף�SiC�����ң�Si3N4����ԭ�Ӿ��壬����C-Si����N-Si������N-Si�����ȶ���Si3N4�е�ߣ�����CCl4��������SiCl4�����Ƿ��Ӿ��壬�ṹ���ƣ���Է�������Խ�е�Խ�ߣ�
��3������������ˮ��Ӧ����HClO��HCl��д��
��4��NCl3�ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ������NO��HNO3��HCl��
����⣺A��B��C��D��E��Ϊ����������Ԫ�أ��ɸ�Ԫ�ص����λ�ÿ�֪��B��C���ڵڶ����ڣ�A��D��E���ڵ������ڣ�BԪ��ԭ�ӵ��������4�����ӣ���BΪ̼Ԫ�أ���CΪ��Ԫ�أ�B��Dͬ���壬��DΪ��Ԫ�أ�Eԭ��������������C��ԭ��������ȣ���E����������Ϊ7����EΪ��Ԫ�أ�
��BΪ̼Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�أ�
��1��BΪ̼Ԫ�ء�CΪ��Ԫ�ء�DΪ��Ԫ�أ�B��D�γɵĻ������ΪSiC��C��D�γɵĻ������ң����߾���ṹ��Ϊ�ռ���״�ṹ�����侧������Ϊԭ�Ӿ��壻�ҵĻ�ѧʽΪSi3N4��
�ʴ�Ϊ��ԭ�Ӿ��壻Si3N4��
��2��BΪ̼Ԫ�ء�DΪ��Ԫ�ء�EΪ��Ԫ�أ�B��D�ֱ���E�γɻ������ΪCCl4����ΪSiCl4��CCl4����������ṹ����Ӧ�Ǽ��Է��ӣ��ף�SiC�����ң�Si3N4����ԭ�Ӿ��壬����C-Si����N-Si������N-Si�����ȶ������Էе��ң��ף�����CCl4��������SiCl4�����Ƿ��Ӿ��壬�ṹ���ƣ���Է�������Խ���Ӽ�������Խǿ���е�Խ�ߣ��е㶡������ԭ�Ӿ���е���ڷ��Ӿ��壬���Էе�Si3N4��SiC��SiCl4��CCl4��
�ʴ�Ϊ���Ǽ��ԣ�Si3N4��SiC��SiCl4��CCl4��
��3��BΪ̼Ԫ�ء�CΪ��Ԫ�أ�B��CԪ���γɵ����ʣ�CN��2Ϊ��±�أ��������������ƣ���CN��2��NaOH��Һ��Ӧ�����ӷ���ʽΪ��CN��2+2OH-=CN-+CNO-+H2O��
�ʴ�Ϊ����CN��2+2OH-=CN-+CNO-+H2O��
��4��CΪ��Ԫ�ء�EΪ��Ԫ�أ������γɵķ��ӵĿռ乹��Ϊ�����Σ������и�ԭ����������8�����ȶ��ṹ��������ΪNCl3��NCl3�ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ�����ɺ�C����ɫ����������ΪNO��C����ۺ�����ΪHNO3��E���⻯��ΪHCl���÷�Ӧ�Ļ�ѧ����ʽΪ3NCl3+5H2O=2NO+HNO3+9HCl��
�ʴ�Ϊ��3NCl3+5H2O=2NO+HNO3+9HCl��
��BΪ̼Ԫ�أ�CΪ��Ԫ�أ�DΪ��Ԫ�أ�EΪ��Ԫ�أ�
��1��BΪ̼Ԫ�ء�CΪ��Ԫ�ء�DΪ��Ԫ�أ�B��D�γɵĻ������ΪSiC��C��D�γɵĻ������ң����߾���ṹ��Ϊ�ռ���״�ṹ�����侧������Ϊԭ�Ӿ��壻�ҵĻ�ѧʽΪSi3N4��
�ʴ�Ϊ��ԭ�Ӿ��壻Si3N4��
��2��BΪ̼Ԫ�ء�DΪ��Ԫ�ء�EΪ��Ԫ�أ�B��D�ֱ���E�γɻ������ΪCCl4����ΪSiCl4��CCl4����������ṹ����Ӧ�Ǽ��Է��ӣ��ף�SiC�����ң�Si3N4����ԭ�Ӿ��壬����C-Si����N-Si������N-Si�����ȶ������Էе��ң��ף�����CCl4��������SiCl4�����Ƿ��Ӿ��壬�ṹ���ƣ���Է�������Խ���Ӽ�������Խǿ���е�Խ�ߣ��е㶡������ԭ�Ӿ���е���ڷ��Ӿ��壬���Էе�Si3N4��SiC��SiCl4��CCl4��
�ʴ�Ϊ���Ǽ��ԣ�Si3N4��SiC��SiCl4��CCl4��
��3��BΪ̼Ԫ�ء�CΪ��Ԫ�أ�B��CԪ���γɵ����ʣ�CN��2Ϊ��±�أ��������������ƣ���CN��2��NaOH��Һ��Ӧ�����ӷ���ʽΪ��CN��2+2OH-=CN-+CNO-+H2O��
�ʴ�Ϊ����CN��2+2OH-=CN-+CNO-+H2O��
��4��CΪ��Ԫ�ء�EΪ��Ԫ�أ������γɵķ��ӵĿռ乹��Ϊ�����Σ������и�ԭ����������8�����ȶ��ṹ��������ΪNCl3��NCl3�ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ�����ɺ�C����ɫ����������ΪNO��C����ۺ�����ΪHNO3��E���⻯��ΪHCl���÷�Ӧ�Ļ�ѧ����ʽΪ3NCl3+5H2O=2NO+HNO3+9HCl��
�ʴ�Ϊ��3NCl3+5H2O=2NO+HNO3+9HCl��
����������ṹ�����ʹ�ϵ��Ԫ�����ڱ���Ԫ�������ɡ���ѧ����ȣ��Ѷ��еȣ���ȷ�ƶ�Ԫ�ص�����Ϊ������Ĺؼ���ע�����֪ʶ�����գ�

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
�����Ŀ