��Ŀ����
�ϳɰ���Ӧ��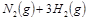
 2NH3(g)���ڹ�ҵ�����еĴ������ã��������˿ڵļ�����������ʳ������ҲΪ���������ṩ���㹻��ԭ�ϡ���Ҫ��ش��������⣺
2NH3(g)���ڹ�ҵ�����еĴ������ã��������˿ڵļ�����������ʳ������ҲΪ���������ṩ���㹻��ԭ�ϡ���Ҫ��ش��������⣺
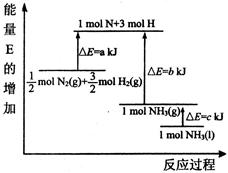
��1���ϳɰ���Ӧ�������仯��ͼ��ʾ����÷�Ӧ���Ȼ�ѧ��ʽΪ����H��ͼ����ĸ��ʾ�� ��
��2�����¶Ⱥ㶨Ϊ298K������㶨Ϊ10L���ܱ������в�úϳɰ���Ӧ�ﵽƽ��ʱ���������������������ʵ����ֱ�Ϊ0.1mol��0.4mol��4mol������¶�������÷�Ӧ��ƽ�ⳣ��K=
��3������£�����22.4mL�İ���ͨ��100mLpHΪ2�������У�����Һ�и�����Ũ�ȵ�˳���ɴ�СΪ ��
��4��������ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4���ù��̵Ļ�ѧ����ʽΪ ���ɴ˿�֪Ksp(CaSO4) Ksp(CaCO3)������ڡ�����С�ڡ����ڡ���
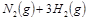
 2NH3(g)���ڹ�ҵ�����еĴ������ã��������˿ڵļ�����������ʳ������ҲΪ���������ṩ���㹻��ԭ�ϡ���Ҫ��ش��������⣺
2NH3(g)���ڹ�ҵ�����еĴ������ã��������˿ڵļ�����������ʳ������ҲΪ���������ṩ���㹻��ԭ�ϡ���Ҫ��ش��������⣺
��1���ϳɰ���Ӧ�������仯��ͼ��ʾ����÷�Ӧ���Ȼ�ѧ��ʽΪ����H��ͼ����ĸ��ʾ�� ��
��2�����¶Ⱥ㶨Ϊ298K������㶨Ϊ10L���ܱ������в�úϳɰ���Ӧ�ﵽƽ��ʱ���������������������ʵ����ֱ�Ϊ0.1mol��0.4mol��4mol������¶�������÷�Ӧ��ƽ�ⳣ��K=
��3������£�����22.4mL�İ���ͨ��100mLpHΪ2�������У�����Һ�и�����Ũ�ȵ�˳���ɴ�СΪ ��
��4��������ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4���ù��̵Ļ�ѧ����ʽΪ ���ɴ˿�֪Ksp(CaSO4) Ksp(CaCO3)������ڡ�����С�ڡ����ڡ���
��9�֣�
 ��1��
��1��

 ��2�֣�
��2�֣�
��2��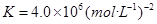 ��2�֣�
��2�֣�
��3��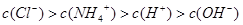 ��2�֣�
��2�֣�
��4��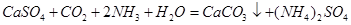 ��2�֣������ڣ�1�֣�
��2�֣������ڣ�1�֣�
 ��1��
��1��

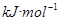 ��2�֣�
��2�֣���2��
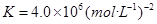 ��2�֣�
��2�֣���3��
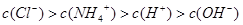 ��2�֣�
��2�֣���4��
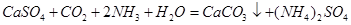 ��2�֣������ڣ�1�֣�
��2�֣������ڣ�1�֣���1����ͼ�ɵø÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪ��



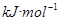
��2��ƽ�ⳣ����ָ���淴Ӧ�ﵽƽ���������Ũ����֮���뷴Ӧ��Ũ����֮���ı�ֵ����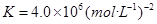 ��
��
��3������£�����22.4mL�İ���ͨ��100mLpHΪ2�������У�����Һ�и�����Ũ�ȵ�˳���ɴ�СΪ��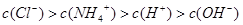 ��
��
��4��������ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4���ù��̵Ļ�ѧ����ʽΪ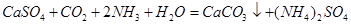 ��
��
�ɴ˿�֪Ksp(CaSO4)����Ksp(CaCO3)



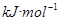
��2��ƽ�ⳣ����ָ���淴Ӧ�ﵽƽ���������Ũ����֮���뷴Ӧ��Ũ����֮���ı�ֵ����
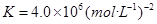 ��
����3������£�����22.4mL�İ���ͨ��100mLpHΪ2�������У�����Һ�и�����Ũ�ȵ�˳���ɴ�СΪ��
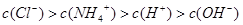 ��
����4��������ͨ��ʢ��CaSO4����Һ�ij������У���ͨ��������CO2�����˺ɵõ���NH4��2SO4���ù��̵Ļ�ѧ����ʽΪ
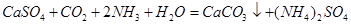 ��
���ɴ˿�֪Ksp(CaSO4)����Ksp(CaCO3)

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
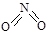 ��
��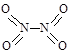 ��ʵ����N��N������Ϊ167kJ��mol��1��NO2�е���˫����ƽ������Ϊ466 kJ��mol��1��N2O4�е���˫����ƽ������Ϊ438.5 kJ��mol��1��
��ʵ����N��N������Ϊ167kJ��mol��1��NO2�е���˫����ƽ������Ϊ466 kJ��mol��1��N2O4�е���˫����ƽ������Ϊ438.5 kJ��mol��1�� N2O4 (g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����( )
N2O4 (g)�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����( )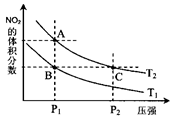
 N2O4��ƽ�ⳣ��K�� �����������С�����䡱����
N2O4��ƽ�ⳣ��K�� �����������С�����䡱����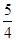 O2��g����
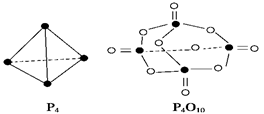
O2��g����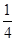 P4O10��s�� ��H����738.5kJ��mol��1
P4O10��s�� ��H����738.5kJ��mol��1 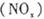 ��������̼������,�������з�����������ʵ�ֽ��ܼ��š��������õȡ�
��������̼������,�������з�����������ʵ�ֽ��ܼ��š��������õȡ�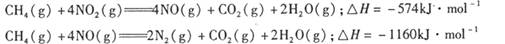
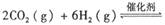
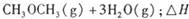 ����֪��һ��ѹǿ��,�÷�Ӧ���¶ȵ����߶�CO2��ת���ʽ��͡���÷�Ӧ��
����֪��һ��ѹǿ��,�÷�Ӧ���¶ȵ����߶�CO2��ת���ʽ��͡���÷�Ӧ�� ________ 0(� >���� <�����������Լ��ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫����ȼ�ϵ��,��õ���и����ĵ缫��Ӧʽ��________________________________,�ŵ��������Һ��PH________ (���������С�����䡱����
________ 0(� >���� <�����������Լ��ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫����ȼ�ϵ��,��õ���и����ĵ缫��Ӧʽ��________________________________,�ŵ��������Һ��PH________ (���������С�����䡱����