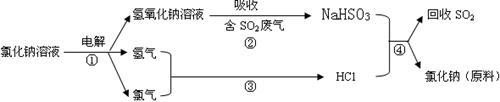
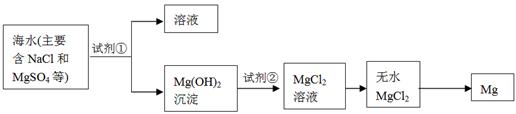
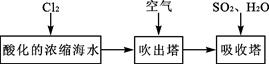
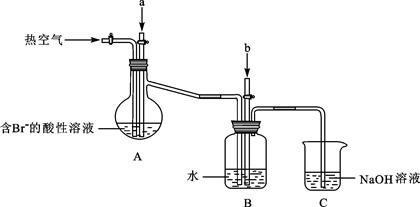
��Ŀ����
��12�֣��ظ����ƣ�Na2Cr2O7���㷺���ںϳ����ϡ�ýȾ���ȣ��Ը�������Ҫ�ɷ�ΪCr2O3������FeO��Al2O3��SiO2�����ʣ�Ϊԭ����ȡ�ظ����Ƶ�����ͼ���£�
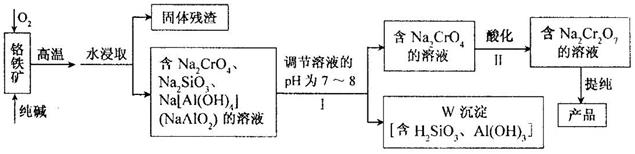
��ش��������⡣
�Ÿ������е�Cr2O3�봿�O2�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ �� ��
��������ֻ��һ���Լ�������Һ��pH��Ӧѡ�� �� �����ţ���
A��ϡ���� B�������ƹ��� C������������Һ
�Ǣ��У�������ҺpH�������Һ��pH��С�����ܵ���W���������ܽ⣬ԭ���ǣ�
�� ���������ӷ���ʽ��ʾ����
�Ȣ��У�Na2CrO4ת��ΪNa2Cr2O7�����ӷ�Ӧ���£�
2CrO42������ɫ����2H�� Cr2O72��(�Ⱥ�ɫ)��H2O
Cr2O72��(�Ⱥ�ɫ)��H2O
�ٸ÷�Ӧ �� ����ǡ����ǡ���������ԭ��Ӧ��
������Na2Cr2O7��Һ���Ⱥ�ɫ���м�������NaOH���壬��Һ �� �����ţ���
A�����ɫ B����ɫ���� C���Ⱥ�ɫ����
����֪��25�棬Ag2CrO4��Ksp��1.12��10��12��Ag2Cr2O7��Ksp��2��10��7��25�棬��Na2Cr2O7��Һ�м���AgNO3��Һ������ֻ����һ��ש��ɫ�������ó����Ļ�ѧʽ�� �� ��

��ش��������⡣
�Ÿ������е�Cr2O3�봿�O2�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ �� ��
��������ֻ��һ���Լ�������Һ��pH��Ӧѡ�� �� �����ţ���
A��ϡ���� B�������ƹ��� C������������Һ
�Ǣ��У�������ҺpH�������Һ��pH��С�����ܵ���W���������ܽ⣬ԭ���ǣ�
�� ���������ӷ���ʽ��ʾ����
�Ȣ��У�Na2CrO4ת��ΪNa2Cr2O7�����ӷ�Ӧ���£�
2CrO42������ɫ����2H��
 Cr2O72��(�Ⱥ�ɫ)��H2O
Cr2O72��(�Ⱥ�ɫ)��H2O�ٸ÷�Ӧ �� ����ǡ����ǡ���������ԭ��Ӧ��
������Na2Cr2O7��Һ���Ⱥ�ɫ���м�������NaOH���壬��Һ �� �����ţ���
A�����ɫ B����ɫ���� C���Ⱥ�ɫ����
����֪��25�棬Ag2CrO4��Ksp��1.12��10��12��Ag2Cr2O7��Ksp��2��10��7��25�棬��Na2Cr2O7��Һ�м���AgNO3��Һ������ֻ����һ��ש��ɫ�������ó����Ļ�ѧʽ�� �� ��
��12�֣�
�� 2Cr2O3+4Na2CO3+3O2 4Na2CrO4+4 CO2��2�֣�
4Na2CrO4+4 CO2��2�֣�
�� A ��2�֣�
�� Al(OH)3��3H����Al3����3H2O ��2�֣�
�Ȣٲ��� ��2�֣� ��A ��2�֣� ��Ag2CrO4 ��2�֣�
�� 2Cr2O3+4Na2CO3+3O2
 4Na2CrO4+4 CO2��2�֣�
4Na2CrO4+4 CO2��2�֣��� A ��2�֣�
�� Al(OH)3��3H����Al3����3H2O ��2�֣�
�Ȣٲ��� ��2�֣� ��A ��2�֣� ��Ag2CrO4 ��2�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ