��Ŀ����
�������ļס��ҡ����������졢��6��ֻ��C��H��O����Ԫ�ص��л���ֱ���ȼ��ʱ�����ĵ�����O2�������ɵ�����ȫ���ֱ�ͨ��������Ũ���ᡢ��ʯ�Һ�Ũ�������ʯ����������֮�Ⱦ�Ϊ9��22����֪��
��6 M���ף�=3 M���ң�=3 M������=3M������=2M���죩=M������=180��
���ҡ����ˮ��Һ��ʹ������Һ��죬�֮����һ���������ܷ���������Ӧ��
����ĺ˴Ź���������ʾ��4�ֲ�ͬ��������ԭ�ӣ�����֮��Ϊ3��1��1��1��
�ܼס��������������ܷ���������Ӧ������������ˮ����������ˮ��
��ش��������⣺
��1���Ľṹ��ʽΪ______�����ж���ͬ���칹�壬����һ�ִ����������ߵ���Һ�У�д����ṹ��ʽ______��
��2���ҡ�����������֮��Ĺ�ϵ��______�����Ľṹ��ʽΪ______��
��3��д��2����������1���ӻ�״���Ļ�ѧ����ʽ______��
��6 M���ף�=3 M���ң�=3 M������=3M������=2M���죩=M������=180��
���ҡ����ˮ��Һ��ʹ������Һ��죬�֮����һ���������ܷ���������Ӧ��
����ĺ˴Ź���������ʾ��4�ֲ�ͬ��������ԭ�ӣ�����֮��Ϊ3��1��1��1��
�ܼס��������������ܷ���������Ӧ������������ˮ����������ˮ��
��ش��������⣺
��1���Ľṹ��ʽΪ______�����ж���ͬ���칹�壬����һ�ִ����������ߵ���Һ�У�д����ṹ��ʽ______��
��2���ҡ�����������֮��Ĺ�ϵ��______�����Ľṹ��ʽΪ______��
��3��д��2����������1���ӻ�״���Ļ�ѧ����ʽ______��
ֻ��C��H��O����Ԫ�ص��л���ֱ���ȼ��ʱ�����ĵ�����O2�������ɵ�����ȫ���ֱ�ͨ��������Ũ���ᡢ��ʯ�Һ�Ũ�������ʯ����������֮�Ⱦ�Ϊ9��22����n��H20����n��CO2��=
��
=1��1�������л����ͨʽΪ��CH2Ox��n����6 M���ף�=3 M���ң�=3 M������=3M������=2M���죩=M������=180��M���ף�=30�����ΪCH2O�����ж���ͬ���칹�壬����һ�ִ����������ߵ���Һ�У���Ϊ�����ǣ������ʽΪC6H12O6�������ҡ����ˮ��Һ��ʹ������Һ��죬�֮����һ���������ܷ���������Ӧ��M���ң�=60������ΪCH3COOH��M���죩=90����ĺ˴Ź���������ʾ��4�ֲ�ͬ��������ԭ�ӣ�����֮��Ϊ3��1��1��1��������Ϊ
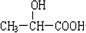
���ס��������������ܷ���������Ӧ������������ˮ����������ˮ����ΪHCOOCH3�����к�-OH����ΪCH2OHCHO��
��1��������������֪����ΪHCHO����ΪCH2OH-CHOH-CHOH-CHOH-CHOH-CHO���ʴ�Ϊ��HCHO��CH2OH-CHOH-CHOH-CHOH-CHOH-CHO��
��2����ΪCH2OHCHO����ΪCH3COOH����ΪHCOOCH3������ʽ��ͬ�����ṹ��ͬ����Ϊͬ���칹�壬�ʴ�Ϊ��ͬ���칹�壻CH2OHCHO��
��3��2����������1���ӻ�״���Ļ�ѧ����ʽΪ
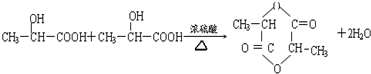
��
�ʴ�Ϊ��

��
| 9 |
| 18 |
| 22 |
| 44 |
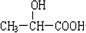
���ס��������������ܷ���������Ӧ������������ˮ����������ˮ����ΪHCOOCH3�����к�-OH����ΪCH2OHCHO��
��1��������������֪����ΪHCHO����ΪCH2OH-CHOH-CHOH-CHOH-CHOH-CHO���ʴ�Ϊ��HCHO��CH2OH-CHOH-CHOH-CHOH-CHOH-CHO��
��2����ΪCH2OHCHO����ΪCH3COOH����ΪHCOOCH3������ʽ��ͬ�����ṹ��ͬ����Ϊͬ���칹�壬�ʴ�Ϊ��ͬ���칹�壻CH2OHCHO��
��3��2����������1���ӻ�״���Ļ�ѧ����ʽΪ

��
�ʴ�Ϊ��

��

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����Ŀ
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʣ��������������������ʾ����ȡ�������ļס��ҡ������Ƴ���ͬ�������Һ���������ʵ����ʵ���Ũ��c���ף���c���ң���c���������������ʣ�������
| ������ | NH4+��Na+��Mg2+ |
| ������ | OH-��NO3-��SO42- |
| A��������NaOH |
| B��������NH4NO3 |
| C����������SO42- |
| D��һ�����ǣ�NH4��2SO4 |

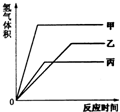 �������ļס��ҡ������ֽ������ֱ�����������������������ͬ��ϡ������ȫ��Ӧ������+2�۵������Σ�����������������ͬ��ͬѹ���뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������
�������ļס��ҡ������ֽ������ֱ�����������������������ͬ��ϡ������ȫ��Ӧ������+2�۵������Σ�����������������ͬ��ͬѹ���뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������