��Ŀ����
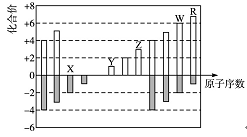
�±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
(1) ����ЩԪ����,��ѧ��������õ���: (�����Ԫ�ط���,��ͬ)��ԭ�ӽṹʾ��ͼΪ________________ ��Ԫ�آ�����Ϊ ������ˮ��Ӧ��ѧ����ʽ ��
(2) ������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��_______��������ǿ�Ļ���
��ĵ���ʽ��:_____________��
(3) �ڵ����������Ļ�ѧʽΪ д��������������ˮ���������������۷�Ӧ�����ӷ���ʽ_____________________________________________��
(4) �õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣� ��
�û��������� (�� �����ۡ������ӡ�)�����
��5����ʾ����ߡ�������γɵĻ�����ĵ���ʽ �� ��
��6���ۡ����⻯��ķе�ߵ� ��ԭ��
��7���ܡ��ޡ����γɵļ����ӵİ뾶��С
��8���ں͢��γɵ�һ�ֶ�Ԫ���������ɫ��ЧӦ������Է���������170��190֮�䣬�Ң���������ԼΪ70�����û�����Ļ�ѧʽΪ_________________��
��9���ܺ͢�����Ԫ���γɺ��зǼ��Լ��Ļ����ﻯѧʽΪ______������û������к���Ԫ�س��÷��� �����øû�������ȡ�۵��ʵĻ�ѧ����ʽ ��
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | | �� | | �� | �� | �� |
| 4 | �� | | | | | | �� | |
(2) ������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��_______��������ǿ�Ļ���
��ĵ���ʽ��:_____________��
(3) �ڵ����������Ļ�ѧʽΪ д��������������ˮ���������������۷�Ӧ�����ӷ���ʽ_____________________________________________��
(4) �õ���ʽ��ʾԪ�آ���Ļ�������γɹ��̣� ��
�û��������� (�� �����ۡ������ӡ�)�����
��5����ʾ����ߡ�������γɵĻ�����ĵ���ʽ �� ��
��6���ۡ����⻯��ķе�ߵ� ��ԭ��
��7���ܡ��ޡ����γɵļ����ӵİ뾶��С
��8���ں͢��γɵ�һ�ֶ�Ԫ���������ɫ��ЧӦ������Է���������170��190֮�䣬�Ң���������ԼΪ70�����û�����Ļ�ѧʽΪ_________________��
��9���ܺ͢�����Ԫ���γɺ��зǼ��Լ��Ļ����ﻯѧʽΪ______������û������к���Ԫ�س��÷��� �����øû�������ȡ�۵��ʵĻ�ѧ����ʽ ��
(19�֣�ÿ��1�֡�)��1��Ar��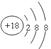 �� �� ��Br2 + H2O =" HBr" + HBrO
�� �� ��Br2 + H2O =" HBr" + HBrO
��2��HClO4��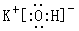 ��3�� N2O5��8H+ + 3Fe + 2NO3��= 3Fe3+ + 2NO�� ��4H2O
��3�� N2O5��8H+ + 3Fe + 2NO3��= 3Fe3+ + 2NO�� ��4H2O
��4��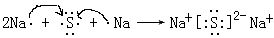 �� ����
�� ����
��5��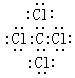 ��
��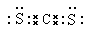 ��6��H2O�е����H2S��H2O����֮�����γ����
��6��H2O�е����H2S��H2O����֮�����γ����
��7��S2- >Cl- >Na+ ��8��S4N4��9��Na2O2 ����ɫ��Ӧ �� 2Na2O2 + 2CO2 ��2Na2CO3 + O2
 �� �� ��Br2 + H2O =" HBr" + HBrO
�� �� ��Br2 + H2O =" HBr" + HBrO��2��HClO4��
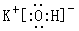 ��3�� N2O5��8H+ + 3Fe + 2NO3��= 3Fe3+ + 2NO�� ��4H2O
��3�� N2O5��8H+ + 3Fe + 2NO3��= 3Fe3+ + 2NO�� ��4H2O��4��
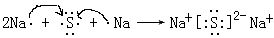 �� ����
�� ������5��
 ��
��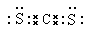 ��6��H2O�е����H2S��H2O����֮�����γ����
��6��H2O�е����H2S��H2O����֮�����γ������7��S2- >Cl- >Na+ ��8��S4N4��9��Na2O2 ����ɫ��Ӧ �� 2Na2O2 + 2CO2 ��2Na2CO3 + O2
���������(1)����Ԫ�������ڱ��е����λ�ÿ�֪������ЩԪ����,��ѧ��������õ���Ar����ԭ�ӽṹʾ��ͼΪ
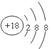 ��Ԫ�آ�����Ϊ�壬�õ�����ˮ��Ӧ��ѧ����ʽ��Br2 + H2O �� HBr + HBrO��
��Ԫ�آ�����Ϊ�壬�õ�����ˮ��Ӧ��ѧ����ʽ��Br2 + H2O �� HBr + HBrO��(2)�ǽ�����Խǿ������������ˮ���������Խǿ������������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��������Խǿ������������ˮ����ļ�СԽǿ���������ǿ�Ļ��������������أ������ʽ��
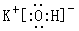 ��
�� (3) ���ǵ�Ԫ�أ�����ǣ�5�ۣ����������������Ļ�ѧʽΪN2O5��������������ˮ���������ᣬ�����������۷�Ӧ�����ӷ���ʽ8H+ + 3Fe + 2NO3��= 3Fe3+ + 2NO�� ��4H2O�� (4) Ԫ�آ�����γɵ����ƣ��������Ӽ��������ӻ�����仯�����õ���ʽ��ʾ�γɹ���
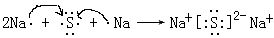 ��
����5������ߡ�������γɵĻ�����ֱ������Ȼ�̼�Ͷ���̼�����Ǻ��й��ۼ��Ĺ��ۻ���������ʽ�ֱ���
 ��
��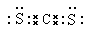 ��
����6������H2O����֮�����γ����������ˮ�ķе����H2S�ķе㡣
��7����������Ų���ͬ�����������뾶��ԭ���������������С�����Ԣܡ��ޡ����γɵļ����ӵİ뾶��С˳����S2- >Cl- >Na+��
��8���ںֱ͢���N��S�����ڶ����γɵĶ�Ԫ���������Է���������170��190֮�䣬�Ң���������ԼΪ70�������Ըû�������Sԭ�ӵĸ���Ӧ���ǽ���
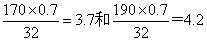 ֮�䣬����Sԭ�ӵĸ�����4������û��������Է���������
֮�䣬����Sԭ�ӵĸ�����4������û��������Է���������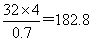 �����Ե�ԭ�ӵĸ�����
�����Ե�ԭ�ӵĸ�����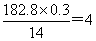 ����˸û�����Ļ�ѧʽΪS4N4��
����˸û�����Ļ�ѧʽΪS4N4����9���ܺ͢�����Ԫ���γɺ��зǼ��Լ��Ļ������ǹ������ƣ��仯ѧʽΪNa2O2������û������к���Ԫ�س��÷�����ɫ��Ӧ�����øû�������ȡ�۵��ʵĻ�ѧ����ʽ2Na2O2 + 2CO2 ��2Na2CO3 + O2��
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij������ͺͿ��㡣����ע�ػ�������������������ͽ��ⷽ����ָ����ѵ����������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ������������ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
�����Ŀ