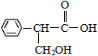
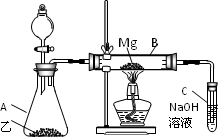
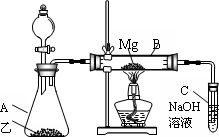
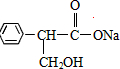��Ŀ����
��ϡH2SO4��NaOH��Һ�ͷ���Ϊԭ����ȡAl(OH)3���ס��ҡ�����λͬѧ���Ʊ�;�����£���Դ˻ش����⣺
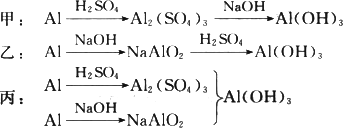
(1)�����������У�������һ���Ļ�ѧ�仯���漰������������Һ�з�����Ӧ��д���û�ѧ��Ӧ�����ӷ���ʽ��________��
(2)�ӻ��ͬ����IJ�Ʒ��ԭ���������ٵĽǶ��������������������������________��������Ϊ��������ķ����У���ȥԭ�����������������⣬���з����������������(��ѡһ������̸һ����Ҫ����)________��
(3)������Ϊ����ķ����У�Ϊ�˴ﵽʹһ�������ķ���������õ�Ŀ�ģ��ڷ���ʵʩ֮ǰ����Ҫ���Ķ���ʵ����________��
�𰸣�
������
��ʾ��
������
|
���� ����(2)��,��������NaOH��Һ�ļ�����������(������H2SO4��Һ�ļ�����������); ����(3)ͨ����������һ������������������1��3�ֿ� ���� |
��ʾ��
|
�������ջ�ѧ��Ӧԭ���ǽ���������Ч;���� |

��ϰ��ϵ�д�
�����Ŀ

 ������Ԫ��A��B��C��D��E��F��ԭ����������������֪����A��Eͬ���壬E�ĵ�����D2��Ӧ������E2D��E2D2���ֹ��壻��F�ĵ�����D2��ȼ�յIJ����ʹƷ����Һ��ɫ��B�ĵ�����D2��ȼ�տ�����BD��BD2�������壻��CA4++DA-=CA3��+A2D�����ַ�Ӧ��������ĵ���������E+��ȣ���ش��������⣺
������Ԫ��A��B��C��D��E��F��ԭ����������������֪����A��Eͬ���壬E�ĵ�����D2��Ӧ������E2D��E2D2���ֹ��壻��F�ĵ�����D2��ȼ�յIJ����ʹƷ����Һ��ɫ��B�ĵ�����D2��ȼ�տ�����BD��BD2�������壻��CA4++DA-=CA3��+A2D�����ַ�Ӧ��������ĵ���������E+��ȣ���ش��������⣺