��Ŀ����
A��B��C��D��EΪ���ֶ�����Ԫ�أ�����A��B��Cλ��ͬһ���ڣ�AԪ�ص���̬�⻯����һ�ֹ㷺Ӧ�õ���������ȼ�ϣ�BԪ�ص��⻯������;��Ϊ�㷺���ܼ���BԪ�ؿɷֱ���A��C��D��E���RB2�ͻ������֪��DB2�У�D��B��������Ϊ7 ��8����EB2�У�E��B��������Ϊ1 ��1���������������ش��������⣺
��1����д��C���ʷ��ӵĵ���ʽ______________��DB2�����л�ѧ��������Ϊ_____________��д��AE2�Ľṹʽ ___________________��
��2��C����̬�⻯����Һ����ԭ����___________________��
��3��C��D��Ͽ�����һ�ֳ�Ӳ���ʣ��йظ��������ʵ������в���ȷ����______________________.
�������� ����ĥ�� �ۿ���ʴ ��ǿ��ԭ�� �ݿ�����
��4��BԪ�ؿɷֱ���A��E�γɶ���������Ԫ�ع��ɵ������ӡ�ij��Һ����������������������ɵ���������Һ��Ϊ�˼������Һ�е������ӣ��ֱ�ȡ������Һ��������ʵ�飺
�ٵ�һ����Һ�м����������ᣬֻ������ɫ���壻
�ڵڶ�����Һ�м���������BaCl2��Һ���а�ɫ�����������ٻ�������������ϡ���ᣬ��ɫ�����������١��ɴ˵ó��Ľ�����_______________________��
��5��EԪ�ص�һ�����������������Ƽ���ˮ����������ԭ��Ӧ������������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ_______________________��������������ˮ��Ӧ�����ӷ���ʽΪ____________________________��
����15�֣�
��1��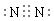 ����2�֣������ԣ����ۼ�����1�֣� S=C=S����2�֣�����5�֣�
����2�֣������ԣ����ۼ�����1�֣� S=C=S����2�֣�����5�֣�
��2�� C���⻯�����֮������������ʹ��е�ϸߣ�2�֣�
��3�� �ڢ� ��2�֣�
��4�� һ����CO32����SO32�� ��SO42�� ������һ��
(����S2O32��һ����������ԣ�����������) ��2�֣�
��5�� SO2 + Na2O2 = Na2SO4 �� SO2 + Cl2 + 2H2O = 4H+ + SO42- + 2Cl-��4�֣�ÿ��2�֣�
����

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д���������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ
��4����֪BA5Ϊ���ӻ����д�������ʽ
��5��DE3����ԭ�ӵ��ӻ���ʽΪ
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ���
 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�

