��Ŀ����
����Ŀ��ijС��ͬѧ������ͼװ�ã��г�������ʡ�ԣ��Ʊ��屽����̽���÷�Ӧԭ����
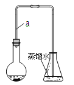
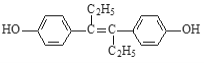
I���Ʊ��屽
��1��װ���г�����a��������______���������壬�Ʊ��屽�ķ�Ӧ����ʽ����______��Ӧ���䷽��ʽΪ________
��2����ʵ������õ��屽Ϊ��ɫ������Ϊ________________��
II�������ᴿ
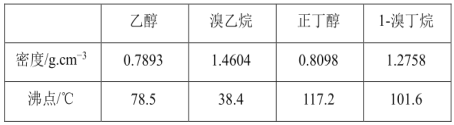
��֪���屽�뱽���ܣ�Һ�塢�����屽�ķе�����Ϊ59�桢80�桢156�档ͬѧ��������������̣�
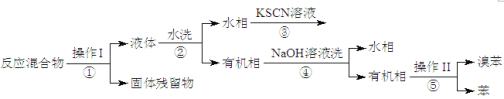
��3��������Ϊ_____________��
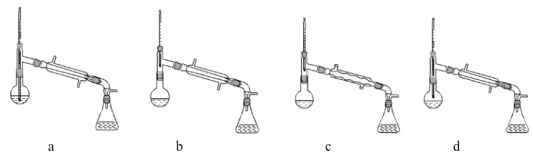
��4�����̢ں͢��У���Ҫ�õ��IJ����������ձ���_______�����̢۵�����Ϊ______�����̢ܵ�������___________��
���𰸡��������������屽 ȡ����Ӧ ![]() +HBr �屽�ܽ��˹������� ���� ��Һ©�� ��Һ��Ѫ��ɫ ��ȥ�屽�е�Br2
+HBr �屽�ܽ��˹������� ���� ��Һ©�� ��Һ��Ѫ��ɫ ��ȥ�屽�е�Br2
��������
��������FeBr3���������Ʊ��屽����Ӧ������к��б����屽���塢FeBr3�Լ�Fe�������̿�֪���������õ����������������ӦΪ���ˣ����������ΪFe�ۣ�Һ���к���FeBr3���塢�屽�����ȣ���ˮϴ��Һ��ˮ�����廯�����л����к��б����屽���壬��������������Һ���ٷ�Һ��ˮ������Ҫ����NaBr��NaBrO�ȣ��л����к��б����屽�����ڶ��߷е㲻ͬ���ɽ���������롣
I����1����ӦΪ���ȷ�Ӧ���������ûӷ��������嵥�ʺͱ�������������ֹ��Ӧ������ӷ����������ܵ�����Ϊ���������������������巢��ȡ����Ӧ�Ʊ��屽������ʽΪ��![]() +HBr���ʴ�Ϊ���������������屽��ȡ����Ӧ��
+HBr���ʴ�Ϊ���������������屽��ȡ����Ӧ��![]() +HBr��
+HBr��
��2���屽����ɫҺ�壬�屽����������ʺ�ɫ���ʴ�Ϊ���屽�ܽ��˹������壻
II����3�������Ϸ�����֪������Ϊ���ˣ�������Ϊ���ʴ�Ϊ������
��4������ˮϴ������NaOH ��Һϴ����õ�ˮ����л��࣬��ӦΪ��Һ����������Ҫ�ձ��⣬����Ҫ��Һ©�������̢ۣ���Һ�к��������ӣ��μ�KCSN��Һ����Һ���ɫ�����̢ܵ������dz�ȥ�屽�л��е��嵥�ʣ��ʴ�Ϊ����Һ©������Һ��Ѫ��ɫ�����ɫ������ȥ�屽�е��塣

 һ����������ϵ�д�
һ����������ϵ�д�����Ŀ��A��B��C��D����ǿ����ʣ�������ˮ�е���ʱ�ɲ�����������(ÿ������ֻ��һ���������һ����ظ�)��
������ | Na����Ba2����NH4+ |
������ | CH3COO����Cl����OH����SO42- |
��֪����A��C��Һ��pH������7����ͬŨ��A��B����Һ��ˮ�ĵ���̶���ͬ����C��Һ��D��Һ����ʱֻ���ɰ�ɫ������B��Һ��C��Һ����ʱֻ���ɴ̼�����ζ�����壬A��Һ��D��Һ���ʱ����������
��1��A��___��B��___��
��2��д��C��D��Ӧ�����ӷ���ʽ___��
��3��25��ʱ��0.1mol��L��1B��Һ��pH��a����B��Һ��c(H��)��c(NH3��H2O)��___(�ú���a�Ĺ�ϵʽ��ʾ)��
��4����������������ʵ���Ũ�ȵ�B��Һ��C��Һ��ϣ���Ӧ����Һ�и�������Ũ���ɴ�С��˳��Ϊ___��
��5����һ�������0.005mol��L��1��C��Һ�У�����һ�������0.00125mol��L��1������ʱ�������Һ��pH��11������Ӧ����Һ���������C��Һ����������֮�ͣ���C��Һ��������������___��