��Ŀ����
��֪�� P4(s)��6Cl2(g) 4PCl3(g) + a kJ�� P4(s)��10Cl2(g)
4PCl3(g) + a kJ�� P4(s)��10Cl2(g) 4PCl5(g) + b kJ��P4������������ṹ��PCl5��P��Cl���ļ���Ϊc kJ/mol��PCl3��P��Cl���ļ���Ϊ1.2c kJ/mol������������ȷ����
4PCl5(g) + b kJ��P4������������ṹ��PCl5��P��Cl���ļ���Ϊc kJ/mol��PCl3��P��Cl���ļ���Ϊ1.2c kJ/mol������������ȷ����
| A��P��P���ļ��ܴ���P��Cl���ļ��� |
B������Cl2(g)��PCl3(g) PCl5(s)�ķ�Ӧ�� PCl5(s)�ķ�Ӧ�� |
C��Cl��Cl���ļ���Ϊ  |
D��P��P���ļ���Ϊ  |
C
�������������A.������PCl5��P��Cl���ļ���Ϊc kJ/mol����PCl3��P��Cl���ļ���Ϊ1.2c kJ/mol�����ʲ�ͬ�������е�P��Cl���ļ��ܲ�ͬ��������Ƚ�P��P���ļ�����P��Cl���ļ��ܵĴ�С������B.��P4(s)��6Cl2(g) 4PCl3(g) + a kJ���� P4(s)��10Cl2(g)
4PCl3(g) + a kJ���� P4(s)��10Cl2(g) 4PCl5(g) + b kJ���ڣ��������ɵ�Cl2(g)��PCl3(g)
4PCl5(g) + b kJ���ڣ��������ɵ�Cl2(g)��PCl3(g) PCl5(g) ��
PCl5(g) ��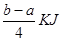 ������û��PCl5(g)
������û��PCl5(g)  PCl5(s)����ЧӦ�����Բ�����Cl2(g)��PCl3(g)
PCl5(s)����ЧӦ�����Բ�����Cl2(g)��PCl3(g) PCl5(s)�ķ�Ӧ�ȡ�����C.���ݷ�Ӧ Cl2(g)��PCl3(g)
PCl5(s)�ķ�Ӧ�ȡ�����C.���ݷ�Ӧ Cl2(g)��PCl3(g) PCl5(g) ��
PCl5(g) ��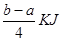 �ķ�Ӧ������ܵĹ�ϵ�ɵ�Cl��Cl��3��1.2c-5��c=
�ķ�Ӧ������ܵĹ�ϵ�ɵ�Cl��Cl��3��1.2c-5��c=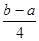 �������ɵ�Cl��Cl�ļ���Ϊ
�������ɵ�Cl��Cl�ļ���Ϊ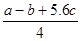 KJ/mol����ȷ��D.���ݼ����뷴Ӧ�ȹ�ϵ ��Cl��Cl�ļ���Ϊ
KJ/mol����ȷ��D.���ݼ����뷴Ӧ�ȹ�ϵ ��Cl��Cl�ļ���Ϊ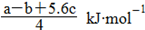 ����ٿɵ�6P��P+6��
����ٿɵ�6P��P+6��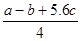 ��4��3��1.2c=a�����P��P���ļ���Ϊ
��4��3��1.2c=a�����P��P���ļ���Ϊ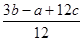 KJ/mol.����
KJ/mol.����
���㣺��������뷴Ӧ�ȡ����ʵĴ���״̬�Ĺ�ϵ��֪ʶ��

����˵�����ʾ������ȷ����
| A�������������������������ֱ���ȫȼ�գ����߷ų������� |
| B���ɡ���(ʯī)����(���ʯ) ��H=1.9kJ/mol����֪�����ʯ��ʯī�ȶ� |
| C����ϡ��Һ�У���+(aq)+OH-(aq)��H2O(l) ��H=-57.3kJ/mol��������1molCH3COOH�뺬��mol NaOH����Һ��ϣ��ų�������С�ڣ���.3kJ |
| D����101kPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2(g)+O2(g)��2H2O(l) ��H=��285.8kJ/mol |
���ȷ�Ӧһ����( )��
| A���ͷ����� |
| B����Ӧ������������������������ |
| C���������� |
| D����Ӧ������������������������ |
���о����ʱ仯ʱ,���ǿ��ԴӲ�ͬ�ĽǶȡ���ͬ�IJ�������ʶ���ʱ仯ʱ������Ļ�ѧ���������仯,�ݴ��ж����������д������( )��
| A����������������Ӧ�����Ȼ��ƺ�,��ṹ���ȶ�����ǿ,��ϵ���������� |
| B������ȼ�տɿ��ɡ����桱�������ڲ�������(��ѧ��)ת��Ϊ�����ͷų��� |
| C���������ڲ������ź�ǿ�Ĺ��ۼ�,��ͨ��״���µ�����ѧ���ʺܻ��� |
| D����Ҫ���Ȳ��ܷ����ķ�Ӧ��һ�������������ķ�Ӧ |
������H2SO4��Һ�м���100mL 0.4 mol��L��1Ba(OH)2��Һ���ų���������5.12 kJ�����������Ba(OH)2��Һ�м���100mL 0.4 mol��L��1HCl��Һʱ���ų�������Ϊ2.2 kJ����Na2SO4��Һ��BaCl2��Һ��Ӧ���Ȼ�ѧ����ʽΪ (����)
| A��Ba2��(aq)��SO42��(aq)=BaSO4(s)����H����2.92 kJ��mol��1 |
| B��Ba2��(aq)��SO42��(aq)=BaSO4(s)����H����18 kJ��mol��1 |
| C��Ba2��(aq)��SO42��(aq)=BaSO4(s)����H����73 kJ��mol��1 |
| D��Ba2��(aq)��SO42��(aq)=BaSO4(s)����H����0.72 kJ��mol��1 |
���й��ڻ�ѧ��Ӧ����������ȷ���� (����)��
| A����Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ |
| B����֪NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l) ��H����57.3 kJ��mol��1����40.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ�Ҳ�ų�57.3 kJ������ |
| C��CO(g)��ȼ������283.0 kJ��mol��1�����ʾCO(g)��ȼ���ȵ��Ȼ�ѧ����ʽΪ2CO(g)��O2(g)=2CO2(g)����H����283.0 kJ��mol��1 |
| D����֪2C(s)��2O2(g)=2CO2(g)����H��a,2C(s)��O2(g)=2CO(g)����H��b����b��a |
ijЩ��ѧ�������������£�
| ��ѧ�� | H��H | Cl��Cl | H��Cl |
| ����/ kJ��mol��1 | 436 | 243 | 431 |
�������Ȼ�ѧ����ʽ����ȷ���� (����)
A.
 H2(g)��
H2(g)�� Cl2(g)=HCl(g) ��H����91.5 kJ��mol��1
Cl2(g)=HCl(g) ��H����91.5 kJ��mol��1B��H2(g)��Cl2(g)=2HCl(g)��H����183 kJ��mol��1
C.
 H2(g)��
H2(g)�� Cl2(g)=HCl(g)��H����91.5 kJ��mol��1
Cl2(g)=HCl(g)��H����91.5 kJ��mol��1D��2HCl(g)=H2(g)��Cl2(g)��H����183 kJ��mol��1
��֪��Ӧ����101 kPaʱ��2C(s)��O2(g)=2CO(g)����H����221 kJ��mol��1
��ϡ��Һ�У�H��(aq)��OH��(aq)=H2O(l)��H����57.3 kJ��mol��1
��H2(g) O2(g)=H2O(g)��H����241.8 kJ��mol��1
O2(g)=H2O(g)��H����241.8 kJ��mol��1
��H2O(g)=H2O(l)��H����44.0 kJ��mol��1
���н�����ȷ����(����)
| A��̼��ȼ���ȴ���110.5 kJ��mol��1 |
| B��Ũ������ϡNaOH��Һ��Ӧ����1 molˮ���ų�57.3 kJ���� |
| C��������ȼ����Ϊ241.8 kJ��mol��1 |
| D��2H2(g)��O2(g)=2H2O(l)�ķ�Ӧ��Ϊ��H����571.6 kJ��mol��1 |