��Ŀ����
����Ŀ����NH3����ԭNxOy�������������������Ⱦ��
��֪����ӦI��4NH3��g����6NO��g��![]() 5N2��g����6H2O��l�� ��H1
5N2��g����6H2O��l�� ��H1
��ӦII��2NO��g����O2��g��![]() 2NO2��g�� ��H2����|��H1|��2|��H2|��
2NO2��g�� ��H2����|��H1|��2|��H2|��
��ӦIII��4NH3��g����6NO2��g��![]() 5N2��g����3O2��g����6H2O��l�� ��H3
5N2��g����3O2��g����6H2O��l�� ��H3
��ӦI�ͷ�ӦII�ڲ�ͬ�¶�ʱ��ƽ�ⳣ�������С��ϵ���±�
�¶�/K | ��ӦI | ��ӦII | ��֪�� K2��K1��K2�䣾K1�� |
298 | K1 | K2 | |
398 | K1�� | K2�� |
��1����H3��_________���æ�H1����H2�Ĵ���ʽ��ʾ�����ƲⷴӦIII��________��Ӧ������ȡ����ȡ�����
��2����ͬ�����£���ӦI��2 L�ܱ������ڣ�ѡ�ò�ͬ�Ĵ�������Ӧ����N2������ʱ��仯��ͼ��ʾ��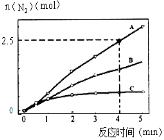
�ټ���0��4������A���������£���Ӧ����v��NO����______________��
������˵������ȷ����__________��
A����λʱ����H��O����N��H�����ѵ���Ŀ���ʱ��˵����Ӧ�Ѿ��ﵽƽ��
B�����ں��ݾ��ȵ��ܱ������з�����Ӧ����Kֵ����ʱ��˵����Ӧ�Ѿ��ﵽƽ��
C���÷�Ӧ�Ļ�ܴ�С˳���ǣ�Ea��A��>Ea��B��>Ea��C��
D������ѹǿ��ʹ��Ӧ���ʼӿ죬����Ϊ�����˻���Ӱٷ���
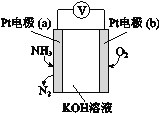
��3���绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ��ʾ��ͼ���ҡ���Һ��OH��缫___________�ƶ�����a��b���������ĵ缫��ӦʽΪ________________��
���𰸡���1����H1��3��H 2��������
��2����0.375 mol��L��1��min��1����CD��
��3��a��2NH3��6e��+6OH����N2+6H2O��
��������
�����������1����4NH3��g����6NO��g��![]() 5N2��g����6H2O��l�� ����2NO��g����O2��g��
5N2��g����6H2O��l�� ����2NO��g����O2��g��![]() 2NO2��g��������3��������4NH3��g����6NO2��g��
2NO2��g��������3�����ó�����4NH3��g����6NO2��g��![]() 5N2��g����3O2��g����6H2O��l�� ��H3=��H1��3��H2����ѧƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬��ӦI�������¶ȵ����ߣ���ѧƽ�ⳣ�����ͣ�������������ԭ���������¶ȣ�ƽ�����淴Ӧ�����ƶ�������Ӧ�����Ƿ��ȷ�Ӧ����H1<0����Ӧ���Ƿ��ȷ�Ӧ����H2<0��|��H1|��2|��H2|���Ƴ���H1��3��H2>0���˷�Ӧ�����ȷ�Ӧ����2�������ݷ�Ӧ���ʵĶ��壬v��N2��=2.5/��4��2��mol/��L��min��=0.3125 mol/��L��min�������ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ���v��NO��=6��v��N2��/5=0.375mol/��L��min��������ͬ���ʵĻ�ѧ��Ӧ���ʱ�ʾ�ﵽƽ�⣬��Ҫ��Ӧ����һ��һ�棬�һ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ�����H��O����˵����Ӧ���淴Ӧ������У�����N��H����Ӧ������Ӧ������У�1molNH3����3molN��H��1molH2O��2molH��O�������ݻ�ѧ��������˵����Ӧ�ﵽƽ�⣬�ʴ���B���˷�Ӧ�Ǿ��ȷ�Ӧ���¶ȸı䣬��ѧƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬���¶Ȳ��䣬��ѧƽ�ⳣ�����䣬��˵���ѧƽ�ⳣ�����䣬˵����Ӧ�ﵽƽ�⣬�ʴ���C��ʹ�ô��������ͻ���ӵĻ�ܣ������Ĵ�Ч��Խ�ã����Խ�ͣ�����ͼ���Ƴ���ܴ�С��E��A��<E��B��<E��C��������ȷ��D������ѹǿ������λ����ڻ���ӵĸ���������ȷ����3��ͨ����һ��Ϊ������ͨ����һ��Ϊ����������ԭ��صĹ���ԭ�������������ƶ����������������ƶ������OH����a���ƶ������ݵ�ع���ԭ���������缫��ӦʽΪ��2NH3��6e��+6OH����N2+6H2O��
5N2��g����3O2��g����6H2O��l�� ��H3=��H1��3��H2����ѧƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬��ӦI�������¶ȵ����ߣ���ѧƽ�ⳣ�����ͣ�������������ԭ���������¶ȣ�ƽ�����淴Ӧ�����ƶ�������Ӧ�����Ƿ��ȷ�Ӧ����H1<0����Ӧ���Ƿ��ȷ�Ӧ����H2<0��|��H1|��2|��H2|���Ƴ���H1��3��H2>0���˷�Ӧ�����ȷ�Ӧ����2�������ݷ�Ӧ���ʵĶ��壬v��N2��=2.5/��4��2��mol/��L��min��=0.3125 mol/��L��min�������ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ���v��NO��=6��v��N2��/5=0.375mol/��L��min��������ͬ���ʵĻ�ѧ��Ӧ���ʱ�ʾ�ﵽƽ�⣬��Ҫ��Ӧ����һ��һ�棬�һ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ�����H��O����˵����Ӧ���淴Ӧ������У�����N��H����Ӧ������Ӧ������У�1molNH3����3molN��H��1molH2O��2molH��O�������ݻ�ѧ��������˵����Ӧ�ﵽƽ�⣬�ʴ���B���˷�Ӧ�Ǿ��ȷ�Ӧ���¶ȸı䣬��ѧƽ�ⳣ��ֻ���¶ȵ�Ӱ�죬���¶Ȳ��䣬��ѧƽ�ⳣ�����䣬��˵���ѧƽ�ⳣ�����䣬˵����Ӧ�ﵽƽ�⣬�ʴ���C��ʹ�ô��������ͻ���ӵĻ�ܣ������Ĵ�Ч��Խ�ã����Խ�ͣ�����ͼ���Ƴ���ܴ�С��E��A��<E��B��<E��C��������ȷ��D������ѹǿ������λ����ڻ���ӵĸ���������ȷ����3��ͨ����һ��Ϊ������ͨ����һ��Ϊ����������ԭ��صĹ���ԭ�������������ƶ����������������ƶ������OH����a���ƶ������ݵ�ع���ԭ���������缫��ӦʽΪ��2NH3��6e��+6OH����N2+6H2O��

 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�