��Ŀ����
12����֪H3PO4 Ϊ���ᣬ������NaH2PO4 ��Һ��pHС��7�����й��ڳ�����0.10mol•L-1 ��NaH2PO4 ��Һ��˵����ȷ���ǣ�������| A�� | c��HPO42-����c��H3PO4�� | B�� | c��Na+��+c��H+��=0.1mol•L-1 | ||
| C�� | c��Na+��=c��H2PO4-��+c��HPO42-��+C��H3PO4�� | D�� | ��ˮϡ�ͺ�n��H+����n��OH-���ij˻����� |
���� A��������NaH2PO4 ��Һ��pHС��7����H2PO4-�ĵ���̶ȴ�����ˮ��̶ȣ���c��HPO42-����c��H3PO4����
B�������Ӳ�ˮ�⣬��c��Na+��=0.1mol•L-1��
C��������Һ��NaH2PO4�������غ��жϣ�
D����ˮϡ�ͺ�H2PO4-�ĵ���̶���������Һ�������ӵ����ʵ�����������Һ��������Ũ�ȼ�С����ˮ�����ӻ����䣬������Һ������������Ũ���������������ӵ����ʵ�������n��H+����n��OH-���ij˻�����
��� �⣺A��H3PO4Ϊ���ᣬ������NaH2PO4 ��Һ��pHС��7��˵��H2PO4-�ĵ���̶ȴ�����ˮ��̶ȣ���c��HPO42-����c��H3PO4������A��ȷ��
B��������0.10mol•L-1��NaH2PO4��Һ�У������Ӳ�ˮ�⣬��c��Na+��=0.1mol•L-1������c��Na+��+c��H+����0.1mol•L-1����B����
C�����������غ�ɵã�c��Na+��=c��H2PO4-��+c��PO43-��+c��HPO42-��+c��H3PO4������C����
D����ˮϡ��NaH2PO4����Һ��H2PO4-�ĵ���̶���������Һ�������ӵ����ʵ���������Һ��������Ũ�Ȼ��С������ˮ�����ӻ������֪��Һ������������Ũ�����������������ӵ����ʵ�������������Һ��n��H+����n��OH-���ij˻�����D����
��ѡA��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ������Ӱ��Ϊ���ؼ���ע�����յ���غ㡢�����غ㼰�ε�ˮ��ԭ�����ж�����Ũ�ȴ�С�е�Ӧ�÷�����

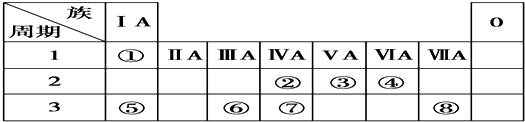
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�| A�� | ��ͭƬ����Ƭ������һ�����ϡ�����У�ͭƬ����������� | |
| B�� | �ں����������п�鱣����Dz��ܸ�ʴ�Dz������������������������� | |
| C�� | ԭ����е����������������������������ƶ� | |
| D�� | �ö��Ե缫���AgNO3��Һһ��ʱ�����һ������Ag�ۣ���Һ�ָܻ�ԭ״ |
| �� | A | ||||||
| B | C | D |
| A�� | Ԫ��C��Ԫ�����ڱ�d�� | |
| B�� | D�ĵ����Ų�ʽΪ1s22s22p63s23p5 | |
| C�� | A�⻯��е����D���⻯��е� | |
| D�� | A��B�������У��뾶��С����B������ |
| A�� | m+n��p+q ����Ӧ�Ƿ��ȷ�Ӧ | B�� | m+n��p+q ����Ӧ�����ȷ�Ӧ | ||
| C�� | m+n��p+q �淴Ӧ�Ƿ��ȷ�Ӧ | D�� | m+n=p+q �淴Ӧ�����ȷ�Ӧ |
| A�� | 150mL 1 mol•L-1��NaCl | B�� | 75mL 1mol•L-1��NH4Cl | ||
| C�� | 150mL 3 mol•L-1��KCl | D�� | 75mL 2 mol•L-1��CaCl2 |
| A�� | CH3COOH?H++CH3COO- | B�� | Ba��OH��2 ?Ba2++2OH- | ||
| C�� | H3PO4 ?3H++PO${\;}_{4}^{3-}$ | D�� | NH3•H2O�TNH${\;}_{4}^{+}$+OH- |
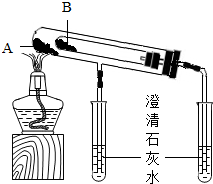 Na2CO3��NaHCO3�����ֳ��������Σ�
Na2CO3��NaHCO3�����ֳ��������Σ�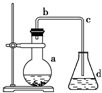 ��ѧʵ��������ͼװ����ȡ�����屽������ƿa��װ���Լ��DZ���Һ������ۣ�d��װ��������ˮ����ش��������⣮
��ѧʵ��������ͼװ����ȡ�����屽������ƿa��װ���Լ��DZ���Һ������ۣ�d��װ��������ˮ����ش��������⣮