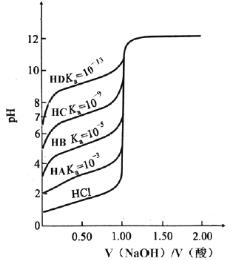
ЬтФПФкШн
ЁОЬтФПЁПСђЫсФјЪЧЕчЖЦФјКЭЛЏбЇЖЦФјЪБЪЙгУЕФжївЊФјбЮЁЃвдЗЯФјДпЛЏМСЃЈжївЊГЩЗжЮЊNiCO3КЭSiO2ЃЌЛЙКЌгаЩйСПFe2O3ЁЂCr2O3ЃЉЮЊдСЯжЦБИСђЫсФјОЇЬхЕФСїГЬШчЭМЫљЪОЃК
вбжЊЃКNi3+ЕФбѕЛЏадБШЯЁHNO3ЧПЁЃ
ЛиД№ЯТСаЮЪЬтЃК
(1)вбжЊЃКCr3++4OH-= CrO2-+2H2OЁЃЁАвЛДЮМюЮіЁБЪБЃЌашМгШыЙ§СПЕФNaOHШмвКЕФФПЕФЪЧ______________________ЁЃ
(2)ЁААБНтЁБЕФФПЕФЮЊ_______________________ЁЃ
(3)ЁАОЛЛЏЁБЁАЙ§ТЫЁБКѓЕУЕНКЌгаСНжждЊЫиЕФВЛШмадЛЏКЯЮяЃЌИУЛЏКЯЮяЕФЛЏбЇЪНЮЊ________ЃЌаДГіЁАбѕЛЏЁБжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЃК____________________________________ЁЃ
(4)ДгNiSO4ШмвКжаЕУЕНСђЫсФјОЇЬхашОЙ§ЁАЯЕСаВйзїЁБЮЊ__________ЃЌ__________ЃЌЙ§ТЫЃЌЯДЕгЃЌИЩдяЃЈЬюВйзїУћГЦЃЉЁЃ
(5)1844ФъЃЌПЦбЇМвЗЂЯжН№ЪєФјПЩвдгУNaH2PO2НЋЫЎШмвКжаЕФNi2+ЛЙдГіРДЃЌNaH2PO2НЋзЊЛЏЮЊH3PO3ЃЌетвЛдРэЯжгУгкЛЏбЇЖЦФјЁЃаДГіЛЏбЇЖЦФјдРэЕФРызгЗНГЬЪН___________ЁЃ
(6)ЮЊВтЖЈСђЫсФјОЇЬхЃЈNiSO4ЁЄn H2OЃЉЕФзщГЩЃЌНјааШчЯТЪЕбщЃКГЦШЁ2.63gбљЦЗЃЌХфГЩ250.00mLШмвКЃЌзМШЗСПШЁХфжЦЕФШмвК25.00mLЃЌгУ0.0400mol/L EDTA(Na2H2Y)БъзМШмвКЕЮЖЈNi2+ЃЈРызгЗНГЬЪНЮЊNi2++H2Y2-=NiY2-+2H+ЃЉЃЌЯћКФEDTAБъзМШмвК25.00mLЁЃдђСђЫсФјОЇЬхЕФЛЏбЇЪНЮЊ_____________________________ЁЃ
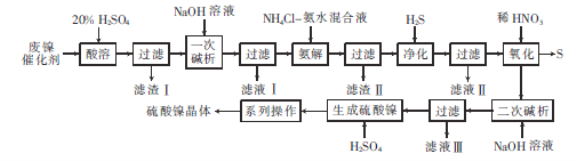
ЁОД№АИЁПГ§ШЅCrдЊЫи ЪЕЯжФјдЊЫигыЬњдЊЫиЕФЗжРы NiS 3NiS+8H++2NO3-=3Ni2++2NOЁќ+3SЁ§4H2O еєЗЂХЈЫѕ РфШДНсОЇ Ni2++H2PO2-+H2O=H3PO3+H++Ni NiSO4Љq6H2O
ЁОНтЮіЁП
ЗЯФјДпЛЏМСЕФжївЊГЩЗжЮЊNiCO3КЭSiO2ЃЌЛЙКЌгаЩйСПFe2O3ЁЂCr2O3ЃЌЫсШмЪБNiCO3ЁЂFe2O3ЁЂCr2O3гыСђЫсЗДгІЩњГЩNiSO4ЁЂFe2(SO4)3ЁЂCr2(SO4)3ЃЌSiO2ВЛЗДгІЃЛЙ§ТЫКѓТЫвКжаМгШыNaOHНјаавЛДЮМюЮіЃЌИљОнЁАCr3++4OH-= CrO2-+2H2OЁБЃЌNiSO4ЁЂFe2(SO4)3зЊЛЏГЩNiЃЈOHЃЉ2ЁЂFeЃЈOHЃЉ3ГСЕэЃЌCrдЊЫизЊЛЏГЩCrO2-НјШыТЫвКIжаЃЛАБНтЙ§ГЬжаNiЃЈOHЃЉ2зЊЛЏГЩ[Ni(NH3)6]2+ЃЛМгШыH2SОЛЛЏЪБ[Ni(NH3)6]2+зЊЛЏЮЊNiSГСЕэЃЌNiSжаМгШыЯЁЯѕЫсЃЌгЩгкNi3+ЕФбѕЛЏадБШЯЁHNO3ЧПЃЌЙЪЯЁЯѕЫсНЋNiSзЊЛЏГЩNi(NO3)2КЭSЃЛЖўДЮМюЮіЪБNi(NO3)2гыNaOHЗДгІЩњГЩNiЃЈOHЃЉ2ГСЕэЃЌNiЃЈOHЃЉ2гыСђЫсЗДгІЩњГЩNiSO4ЃЌСђЫсФјШмвКОеєЗЂХЈЫѕЁЂРфШДНсОЇЁЂЙ§ТЫЁЂЯДЕгЁЂИЩдяЕУСђЫсФјОЇЬхЃЛОнДЫЗжЮізїД№ЁЃ
ЃЈ1ЃЉИљОнЁАCr3++4OH-= CrO2-+2H2OЁБЃЌЁАвЛДЮМюЮіЁБЪБМгШыЙ§СПЕФNaOHШмвКЃЌНЋCr3+КЭЧтбѕИљЗДгІЩњГЩСЫCrO2-ЃЌГ§ШЅСЫCrдЊЫиЃЌЙЪБОЬтД№АИЃКГ§ШЅCrдЊЫиЁЃ
ЃЈ2ЃЉАБНтЙ§ГЬжаМгШыNH4ClЁЊАБЫЎЛьКЯвКЃЌNiЃЈOHЃЉ2зЊЛЏГЩ[Ni(NH3)6]2+ЃЌЖјВЛШмНтFeЃЈOHЃЉ3ЃЌДгЖјЪЕЯжФјдЊЫиКЭЬњдЊЫиЕФЗжРыЃЌБОЬтД№АИЮЊЃКЪЕЯжФјдЊЫиКЭЬњдЊЫиЕФЗжРыЁЃ
ЃЈ3ЃЉЁАОЛЛЏЁБЙ§ГЬжаЭЈШыH2SЃЌЩњГЩNiSГСЕэЃЛЁАбѕЛЏЁБЙ§ГЬжаЃЌвђЮЊNi3+ЕФбѕЛЏадБШЯЁHNO3ЧПЃЌЯЁHNO3БЛЛЙдЮЊNOЃЌNiSБЛбѕЛЏЮЊSЃЌЗДгІЕФРызгЗНГЬЪНЮЊ3NiS+8H++2NO3-=3Ni2++2NOЁќ+3SЁ§4H2OЃЌЙЪБОЬтД№АИЮЊЃКNiSЃЛ3NiS+8H++2NO3-=3Ni2++2NOЁќ+3SЁ§4H2OЁЃ
ЃЈ4ЃЉДгNiSO4ШмвКжаЕУЕНСђЫсФјОЇЬхашОЙ§ЁАЯЕСаВйзїЁБЮЊеєЗЂХЈЫѕЁЂРфШДНсОЇЁЂЙ§ТЫЁЂЯДЕгЁЂИЩдяЃЌЙЪБОЬтД№АИЮЊЃКеєЗЂХЈЫѕЃЛРфШДНсОЇЁЃ
ЃЈ5ЃЉгУNaH2PO2НЋЫЎШмвКжаЕФNi2+ЛЙдГіРДЃЌNaH2PO2НЋзЊЛЏЮЊH3PO3ЃЌNiдЊЫиЕФЛЏКЯМлгЩ+2МлНЕЮЊ0МлЃЌPдЊЫиЕФЛЏКЯМлгЩ+1МлЩ§жС+3МлЃЌИљОнЕУЪЇЕчзгЪиКуЁЂдзгЪиКуКЭЕчКЩЪиКуЃЌдђРызгЗНГЬЪНЮЊЃКNi2++H2PO2-+H2O=H3PO3+H++NiЃЌЙЪБОЬтД№АИЮЊЃКNi2++H2PO2-+H2O=H3PO3+H++NiЁЃ
ЃЈ6ЃЉИљОнЗНГЬЪНNi2++H2Y2-=NiY2-+2H+ПЩжЊЃЌдбљЦЗжаКЌNiSO4ЕФЮяжЪЕФСПЮЊЃК0.0400mol/LЁС0.025LЁС10=0.01molЃЌИУЮяжЪЕФЕФФІЖћжЪСПЮЊ![]() =263g/molЃЌНсОЇЫЎЕФЯЕЪ§nЮЊ
=263g/molЃЌНсОЇЫЎЕФЯЕЪ§nЮЊ![]() =6ЃЌдђЛЏбЇЪНЮЊЃКNiSO4Љq6H2OЃЌБОЬтД№АИЮЊЃКNiSO4Љq6H2OЁЃ
=6ЃЌдђЛЏбЇЪНЮЊЃКNiSO4Љq6H2OЃЌБОЬтД№АИЮЊЃКNiSO4Љq6H2OЁЃ

 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИЁОЬтФПЁПФГаЁзщЭЌбЇЭЈЙ§ЪЕбщбаОПFeCl3ШмвКгыCuЗлЗЂЩњЕФбѕЛЏЛЙдЗДгІЁЃЪЕбщМЧТМШчЯТЃК
ађКХ | I | II | III |
ЪЕбщВНжш |
ГфЗжеёЕДЃЌМгШы2mLеєСѓЫЎ |
ГфЗжеёЕДЃЌМгШы2mLеєСѓЫЎ |
ГфЗжеёЕДЃЌМгШы2mLеєСѓЫЎ |
ЪЕбщЯжЯѓ | ЭЗлЯћЪЇЃЌШмвКЛЦЩЋБфЧГЃЌМгШыеєСѓЫЎКѓЮоУїЯдЯжЯѓ | ЭЗлгаЪЃгрЃЌШмвКЛЦЩЋЭЪШЅЃЌМгШыеєСѓЫЎКѓЩњГЩАзЩЋГСЕэ | ЭЗлгаЪЃгрЃЌШмвКЛЦЩЋЭЪШЅЃЌБфГЩРЖЩЋЃЌМгШыеєСѓЫЎКѓЮоАзЩЋГСЕэ |
ЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
A. ЪЕбщIЁЂIIЁЂIIIжаОљЩцМАFe3+БЛЛЙд
B. ЖдБШЪЕбщIЁЂIIЫЕУїАзЩЋГСЕэЕФВњЩњгыЭЗлЕФСПгаЙи
C. ЪЕбщIIЁЂIIIжаМгШыеєСѓЫЎКѓc(Cu2+)ЯрЭЌ
D. ЯђЪЕбщIIIЗДгІКѓЕФШмвКжаМгШыБЅКЭNaClШмвКПЩФмГіЯжАзЩЋГСЕэ