��Ŀ����
��14�֣���ѧʵ�����о��������ʵĻ�����
��1�������й�ʵ�������������ݺ������� ������ţ���
a.������������CuSO4��5H2O����ⶨ�ᾧˮ��������
b.�ø����pH��ֽ�ⶨŨ�����pH
c.�ù��Ϊ20mL����Ͳ����ȡ16.8mL��Na2CO3��Һ
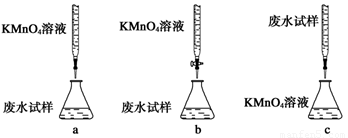
��2��ij��ˮ��Ʒ�к���һ����Na+��CO32-��SO32-���ס������о�С�����ⶨ����SO32-���ӵ�Ũ�ȡ�
���鷽����
�Լ�X�������Լ���ѡ��
a.0.1mol��L-1KMnO4(H2SO4�ữ)��Һb.0.5mol��L-1NaOH��Һc.������ˮd.KI��Һ
�ټ�����Լ�XΪ ������ĸ��ţ�������SO42-��Ҫ�����ӷ���ʽΪ ��
�ڼ��鷽���У���iii���ġ�ϵ�в����������IJ������Ƹ�Ϊ ��
���鷽����
i.���ձ�ʢȡ��ˮ����������������̿����ȥ��ˮ����ɫ�����ˣ�ȡ��Һ��
ii.��ȷ��ȡ20.00mL���˺��ˮ������ѡ��ʹ����ɫ��0.1 mol��L-1KMnO4��H2SO4�ữ����Һ���еζ������йط�ӦΪ��2MnO4-+5SO32-+6H+=2Mn2++5SO42-+3H2O
iii.��¼���ݣ����㡣
��������Ƶ����еζ���ʽ�У���������� ���гֲ�����ȥ��������ĸ��ţ�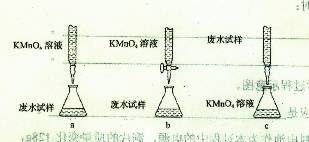
��3��ijͬѧ�Ʊ�Fe(OH)3���壺�ýྻ�ձ���ȡ��������ˮ���������ڣ����ձ��л����μӱ��͵�FeCl3��Һ���������ò����������������Һ����ǡ���ͬѧ�Ʊ�����ʧ�ܵ�ԭ���� ������Ϊ�ɹ��Ƶ�Fe(OH)3��������������� ��
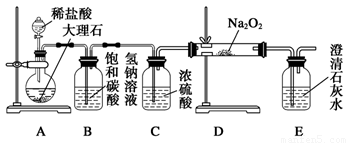
��4������ͼװ�ý���CO2���ʵ��й�ʵ�顣
���Լ�ƿB��ʢ�б���NaHCO3��Һ����Ŀ���� ��
�ڷ�Ӧ�����У�E�г���ʯ��ˮ����ǣ�E�еĻ����ϵ�г����ڵ���ƽ�⡢ˮ��ƽ���⣬�������ܽ�ƽ�⣬�÷���ʽ��ʾ���ܽ�ƽ���ϵ ��

����