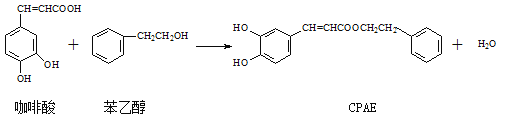
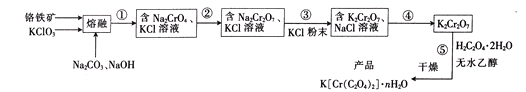
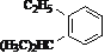��Ŀ����
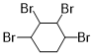
����Ŀ��1��4�����Ҷ�����ͨ������·�ߺϳɣ�ijЩ��Ӧ�ķ�Ӧ��ͷ�Ӧ����δ�г�����
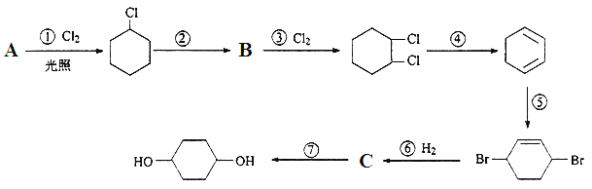
1��4�����Ҷ���
��1��A�Ľṹ��ʽ��___________________________��
��2����Ӧ��Ӧ������Լ���������_______________________________��
��3��д����Ӧ�������Ļ�ѧ����ʽ��
��____________________________________________��
��_________________________________________��
��4�����ķ�Ӧ������_________�����ķ�Ӧ������_________�������߸���Ӧ�����ڼӳɷ�Ӧ����_________���Ӧ��ţ���
��5����Ӧ���п��ܲ���һ�������л����������ܵĽṹ��ʽΪ________________________����д��һ�ּ��ɣ�
���𰸡�![]() NaOH�Ĵ���Һ������
NaOH�Ĵ���Һ������ ![]() +2NaOH
+2NaOH![]()
![]() +2NaCl+2H2O
+2NaCl+2H2O ![]() +2NaOH
+2NaOH![]()
![]() +2NaBr ȡ����Ӧ ��ȥ��Ӧ �ۢݢ�
+2NaBr ȡ����Ӧ ��ȥ��Ӧ �ۢݢ� 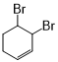 ��
��
��������
��ת����ϵ��֪AΪ![]() ��BΪ
��BΪ![]() ��CΪ
��CΪ![]() ������л���Ľṹ�������Լ���ĿҪ������⡣
������л���Ľṹ�������Լ���ĿҪ������⡣
��1��������������֪��A�Ľṹ��ʽΪ��![]() ��
��
��2����Ӧ��Ϊ![]() ��
��![]() �������ķ�ӦΪ±��������ȥ��Ӧ���䷴Ӧ����Ϊ��NaOH�Ĵ���Һ�����ȣ�
�������ķ�ӦΪ±��������ȥ��Ӧ���䷴Ӧ����Ϊ��NaOH�Ĵ���Һ�����ȣ�
��3����Ϊ![]() ��NaOH����Һ�м��ȷ�����ȥ��Ӧ����Ӧ�ķ���ʽΪ��
��NaOH����Һ�м��ȷ�����ȥ��Ӧ����Ӧ�ķ���ʽΪ��![]() +2NaOH
+2NaOH![]()
![]() +2NaCl+2H2O��
+2NaCl+2H2O��
��Ϊ![]() ��NaOH��Һ�з���ȡ����Ӧ����Ӧ�ķ���ʽΪ��
��NaOH��Һ�з���ȡ����Ӧ����Ӧ�ķ���ʽΪ��![]() +2NaOH
+2NaOH![]()
![]() +2NaBr��
+2NaBr��
��4���ɷ�Ӧ���������ʵ�ת����֪����Ӧ��Ϊȡ����Ӧ����Ϊ��ȥ��Ӧ����Ϊ�ӳɷ�Ӧ����Ϊ��ȥ��Ӧ���ݼӳɷ�Ӧ����Ϊ�ӳɷ�Ӧ����Ϊȡ����Ӧ��
��5��������Ӧ��ʱ��![]() �������巢��1��2�ӳɻ�1��4�ӳɣ�Ҳ����2mol����ȫ�����ӳɷ�Ӧ������������֮�⣬����������
�������巢��1��2�ӳɻ�1��4�ӳɣ�Ҳ����2mol����ȫ�����ӳɷ�Ӧ������������֮�⣬���������� ��
�� ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�