��Ŀ����
�Ķ��������ݣ��ش����⡣
ұ������һ�����������ַ������ٽ�̿������ˮú��(��H2����CO)�����ۻ��ý����û������ܵ�ⷨ�����ַ���������ȱ�㣬�ڹ�ҵ�Ͼ���Ӧ�á�
(1)һ����ɫ����A����ȵ�̿��Ӧ���õ���һ����ɫ����B��B�����ȵ�����ͭ��Ӧ���ֵõ�A����A��B�ֱ�Ϊ_______(�����)��
A.O2��CO2 B.O2��CO C.CO2��CO D.CO��CO2
(2)����˵���������_______(�����)��
A.�ԷϾɽ�������ô��������ǻ���������
B.��������Ҫ������ʯ�ĸ�����ұ������������
C.���ý�����ұ������ͨ�����������Һ�Ƶ�
D.�Ȼ�ԭ���л�ԭ���н�̿��һ����̼����������ý�����
(3)��(Ti)�С�δ��������֮�ơ���ҵ�ϳ��ԣ�TiCl4+2Mg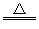 Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��
Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��
A.ϡ�������� B.������ C.������ D.CO2������
ұ������һ�����������ַ������ٽ�̿������ˮú��(��H2����CO)�����ۻ��ý����û������ܵ�ⷨ�����ַ���������ȱ�㣬�ڹ�ҵ�Ͼ���Ӧ�á�
(1)һ����ɫ����A����ȵ�̿��Ӧ���õ���һ����ɫ����B��B�����ȵ�����ͭ��Ӧ���ֵõ�A����A��B�ֱ�Ϊ_______(�����)��
A.O2��CO2 B.O2��CO C.CO2��CO D.CO��CO2
(2)����˵���������_______(�����)��
A.�ԷϾɽ�������ô��������ǻ���������
B.��������Ҫ������ʯ�ĸ�����ұ������������
C.���ý�����ұ������ͨ�����������Һ�Ƶ�
D.�Ȼ�ԭ���л�ԭ���н�̿��һ����̼����������ý�����
(3)��(Ti)�С�δ��������֮�ơ���ҵ�ϳ��ԣ�TiCl4+2Mg
 Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��
Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��A.ϡ�������� B.������ C.������ D.CO2������
(1)C (2)C (3)A
(1)CO���л�ԭ�ԣ��ɻ�ԭCuO�õ�Cu��(2)����Na��Mg��Al�Ȼ��ý�������ͨ�����������Һ�Ƶã����ǵ������̬������������Ƶã�(3)Mg����O2��N2��CO2��Ӧ���ʸ÷�Ӧ����ϡ�������н���

��ϰ��ϵ�д�
�����Ŀ
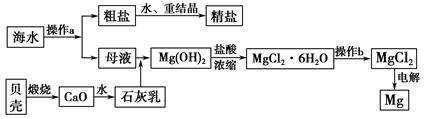
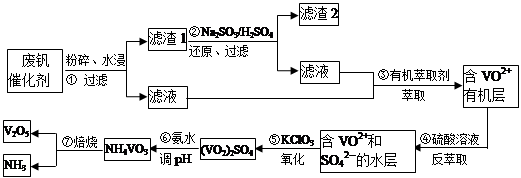
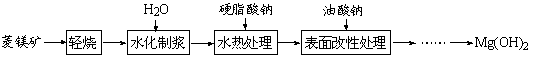
 VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
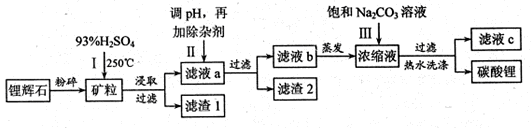
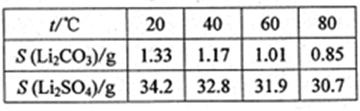
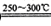 Li2SO4+Al2O3·4SiO2?H2O
Li2SO4+Al2O3·4SiO2?H2O