��Ŀ����
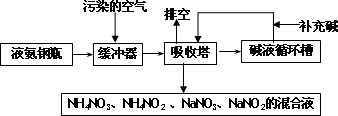
ij���᳧�����Ŀ�����Ҫ��Ⱦ��Ϊ���������Ϊ�˱����������ۺ����ã��ɲ��ð�-���������շ����˷����м����պͰ������������ŵ㣬�����չ����������£�
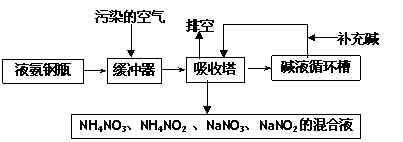 |
��1���ſ����ʵ���Ҫ�ɷ�Ϊ____________________________��
��2��������������ǰ��һ����������Ŀ����________________________________��
��3�����������ų��Ļ��Һ��;֮һΪ________________ ��
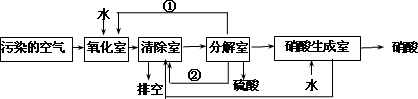
��������Ҫ��Ⱦ��Ϊ���������͵���������о���Ա�����ͬʱ���������ж�������͵���������ķ���������ת��Ϊ��������ᣬ�����������£�
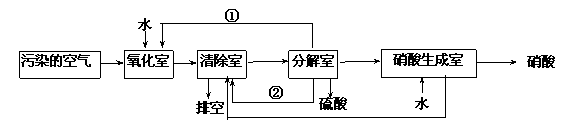
�����з����ķ�Ӧ���£�
a.�����ң�NO2(g) + SO2(g) + H2O (l)=H2SO4 (l)+ NO(g)��H=a kJ��mol-1
b.����ң�NO (g)+ NO2 (g)= N2O3(g) ��H= b kJ��mol-1
N2O3 (g)+ 2H2SO4(l) = 2NOHSO4 (s)+ H2O(l) ��H=c kJ��mol-1
c.�ֽ��ң�4NOHSO4 (s)+ O2 (g)+ 2H2O (l)= 4H2SO4 (l)+ 4NO2(g) ��H= dkJ��mol-1
�ش��������⣺
��4���ٺ͢ڷֱ�Ϊ��д��ѧʽ��______________��_____________��
��5��д��SO2�� O2 �� H2O��Ӧ����H2SO4���Ȼ�ѧ����ʽ��
____________________________________________________________��
��1��N2�� O2 ��2�֣�ֻ��N2Ҳ��ԣ�
��2��ʹ�����ʹ�����ֻ�� ��2�֣�
��3������ ��1�֣�
��4��NO2 H2SO4 ��2�֣�
��5��2SO2(g)+ O2(g) +2H2O(l)= 2H2SO4(l) ��H=(2a+2b+2c+d) kJ��mol-1 ��3�֣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�