��Ŀ����
ij��ѧ�С��Ϊ̽����������ȡ��������Ϊԭ�Ͻ����ض���Ӧ������������ʵ��װ�á�
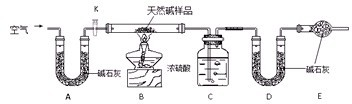
(1)Ϊ�����װ�õ������ԣ�������ͬѧ�ֱ��������������������
��ͬѧ����A�����ȣ�Ȼ����������Եļ������۲�B��C��E�е��й���������жϡ�
��ͬѧ���ȹر�K����ȼA���ľƾ��ƣ��۲�B�е��й���������жϣ��ٴ�K����ȼD���ľƾ��ƣ�����C��E�е��й���������жϡ�
�����____________________________(��ס����ҡ�)ͬѧ���������á�
(2)ʵ�鿪ʼʱ���ȵ�ȼA���ľƾ��ƣ�������K����Cl2��������װ�ã��ٵ�ȼD���ƾ��ƣ�������Eװ�á�Cl2ͨ��Cƿ���ٽ���D��Dװ�õ�Ӳ�ʲ������ڷ�����Ӧ�������ΪCO2��HCl��
��д��D�з�Ӧ�Ļ�ѧ����ʽ��____________________��װ��C��������____________________________��
(3)��E������ɫʯ����Һ����ɫ�ȱ�Ϊ��ɫ���ٱ�Ϊ��ɫ����ԭ����______________��
(4)����E���Թ�����Һ��Ϊ����ʯ��ˮ����Ӧ������______________(��С����ޡ�)��ɫ���������������������Ҫԭ����(�û�ѧ����ʽ��ʾ)______________��
(5)D����Ӧ��Ϻر�K����ȥ�ƾ��ƣ��������ȵ����ã�A������Cl2��������ʱB�е�������______________��B��������____________________________________��
(6)��ʵ��װ�õ�һ������ȱ����__________________________________________��
(1)��
(2)2Cl2+C+2H2O![]() CO2+4HCl �ṩˮ����
CO2+4HCl �ṩˮ����
(3)HCl����ˮʹʯ����ɫ��������������ˮ����HClO��ʹʯ����ɫ
(4)�� Ca(OH)2+2HCl![]() CaCl2+2H2O
CaCl2+2H2O
(5)����©����Һ��������ƿ��Һ���½� ������������������������Ⱦ����
(6)��β������װ��
������(2)Cl2����C�����ˮ������һ�����D�з�Ӧ��
2Cl2+C+2H2O![]() CO2+4HCl��C���������ṩˮ������
CO2+4HCl��C���������ṩˮ������
(3)HCl����ˮʹʯ���죻δ��Ӧ��Cl2��ˮ��Ӧ���ɲ���HClO��ʹʯ����ɫ��
(4)������Ca(OH)2+2HCl![]() CaCl2+2H2O��
CaCl2+2H2O��
(5)����©����Һ��������Bƿ��Һ���½���
Bƿ������������������������Cl2��Ⱦ������
(6)�����ж���Ӧ���β������װ�á�

��A��B��C��D��E��5ֻС��ƿ�зֱ��������ϸ��˿������ʳ��ˮ��ϸ��˿��������ˮ��ϸ��˿����ʳ��ˮ��ȫ��û��ϸ��˿�Լ�����ˮ��ȫ��û��ϸ��˿��Ȼ��װ�����ͼ15-34��ʾ��5��װ�ã�ÿ��һ��ʱ�����������ˮ�������ĸ߶ȣ�������±���ʾ��������������Ϊ����ˮ�������ĸ߶ȣ���λ��cm):

ͼ15-34
ʱ��/h | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
Aƿ��ʢ������˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Bƿ��ʢմ��ʳ��ˮ����˿�� | 0 | 0.4 | 1.2 | 3.4 | 5.6 | 7.6 | 9.8 |
Cƿ��ʢմ����ˮ����˿�� | 0 | 0 | 0 | 0.3 | 0.8 | 2.0 | 3.5 |
Dƿ��ʢ��ȫ��û��ʳ��ˮ�е���˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Eƿ��ʢ��ȫ��û����ˮ�е���˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
��1���������Ϊ��̽�������⣬��Ľ����ǣ�д�������ƣ�������������������������������?
��2��ʵ��ǰ����μ����ʵ��װ�õ������ԣ�����������������������������������������
��3��ͨ��ʵ��õ������ݣ����Եõ��Ľ���������������������������������������������