��Ŀ����
 ��R��R�䡢R���Ǹ�������������ʽϩ����������Ӧ��˫�����������������Һ���������ѣ��ڶϼ����˵�̼ԭ�Ӷ���������Ϊ�Ȼ���ͪ�ʻ������������õIJ�������ƲⷴӦ��ϩ���Ľṹ������A��B��C��������˫���Ļ�������DZ�������Һ������
��R��R�䡢R���Ǹ�������������ʽϩ����������Ӧ��˫�����������������Һ���������ѣ��ڶϼ����˵�̼ԭ�Ӷ���������Ϊ�Ȼ���ͪ�ʻ������������õIJ�������ƲⷴӦ��ϩ���Ľṹ������A��B��C��������˫���Ļ�������DZ�������Һ������1molA������ʽC8H16���������õ�2molͪD��
1molB������ʽC8H14���������õ�2molͪE��1mol��Ԫ���ᣮ
1molC������ʽC8H14��������ֻ�õ�һ�ֲ������һ��û��֧���Ķ�Ԫ���ᣮ
��ݴ��ƶ�B��C��D��E�Ľṹ��ʽΪ��B______��C______��D______��E______��
���𰸡��������ɷ�Ӧ��Ϣ��֪��˫�����������������Һ���������ѣ���������Cԭ�Ӻ���Hԭ�ӣ��������������ᣬ���ϼ�����̼ԭ�Ӷ���������Ϊ�Ȼ�������������Cԭ��û��Hԭ�ӣ���ϼ�����̼ԭ�Ӷ���������Ϊͪ�ʻ�������ͪ��
1molA������ʽC8H16���������õ�2molͪD��A�к���1��C=C˫����Ϊ�Գƽṹ��������Cԭ����û��Hԭ�ӣ�AΪ��
CH3CH2C��CH3��=C��CH3��CH2CH3�������Ϣ�ж�D�Ľṹ��
1molB������ʽC8H14���������õ�2molͪE��1mol��Ԫ���ᣬ�����к���2��C=C˫������Ϊ�Գƽṹ����BΪ����CH3��2C=CH-CH=C��CH3��2�������Ϣ�ж�E�Ľṹ��
1molC������ʽC8H14��������ֻ�õ�һ�ֲ������һ��û��֧���Ķ�Ԫ���ᣬ��CΪ��ϩ���ݴ���дC�Ľṹ��ʽ��
����⣺1molA������ʽC8H16���������õ�2molͪD��A�к���1��C=C˫����Ϊ�Գƽṹ��������Cԭ����û��Hԭ�ӣ�AΪ��CH3CH2C��CH3��=C��CH3��CH2CH3�������Ϣ��֪��DΪCH3COCH2CH3��
1molB������ʽC8H14���������õ�2molͪE��1mol��Ԫ���ᣬ�����к���2��C=C˫������Ϊ�Գƽṹ����BΪ����CH3��2C=CH-CH=C��CH3��2�������Ϣ��֪EΪ��CH3��2C=O��
1molC������ʽC8H14��������ֻ�õ�һ�ֲ������һ��û��֧���Ķ�Ԫ���ᣬ��CΪ��ϩ��C�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ����CH3��2C=CH-CH=C��CH3��2�� ��CH3COCH2CH3����CH3��2C=O��
��CH3COCH2CH3����CH3��2C=O��
�����������л�����ƶϣ��ѶȲ�������Ӧ��Ϣ�ǽ���Ĺؼ����ܽϺõĿ���ѧ�����Ķ���������˼ά���������ȵ����ͣ�
1molA������ʽC8H16���������õ�2molͪD��A�к���1��C=C˫����Ϊ�Գƽṹ��������Cԭ����û��Hԭ�ӣ�AΪ��
CH3CH2C��CH3��=C��CH3��CH2CH3�������Ϣ�ж�D�Ľṹ��
1molB������ʽC8H14���������õ�2molͪE��1mol��Ԫ���ᣬ�����к���2��C=C˫������Ϊ�Գƽṹ����BΪ����CH3��2C=CH-CH=C��CH3��2�������Ϣ�ж�E�Ľṹ��
1molC������ʽC8H14��������ֻ�õ�һ�ֲ������һ��û��֧���Ķ�Ԫ���ᣬ��CΪ��ϩ���ݴ���дC�Ľṹ��ʽ��
����⣺1molA������ʽC8H16���������õ�2molͪD��A�к���1��C=C˫����Ϊ�Գƽṹ��������Cԭ����û��Hԭ�ӣ�AΪ��CH3CH2C��CH3��=C��CH3��CH2CH3�������Ϣ��֪��DΪCH3COCH2CH3��
1molB������ʽC8H14���������õ�2molͪE��1mol��Ԫ���ᣬ�����к���2��C=C˫������Ϊ�Գƽṹ����BΪ����CH3��2C=CH-CH=C��CH3��2�������Ϣ��֪EΪ��CH3��2C=O��
1molC������ʽC8H14��������ֻ�õ�һ�ֲ������һ��û��֧���Ķ�Ԫ���ᣬ��CΪ��ϩ��C�Ľṹ��ʽΪ
 ��
���ʴ�Ϊ����CH3��2C=CH-CH=C��CH3��2��
 ��CH3COCH2CH3����CH3��2C=O��
��CH3COCH2CH3����CH3��2C=O�������������л�����ƶϣ��ѶȲ�������Ӧ��Ϣ�ǽ���Ĺؼ����ܽϺõĿ���ѧ�����Ķ���������˼ά���������ȵ����ͣ�

��ϰ��ϵ�д�
 ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д� ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ
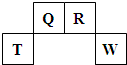 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�