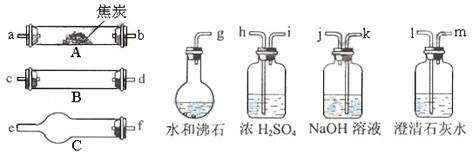
��Ŀ����
ij������ 2mol/LNaOH��Һ����0.1mol/LNaOH��Һ100 mL�����������ɸ�ʵ�飺��1������Ӧ��ѡ�õ�ʵ�������� ��
��2��ʵ����������ȡ2mol/LNaOH��Һ�����Ϊ_______________��
��3����������ȡ��NaOH��Һ����С�ձ��м�ˮϡ����ȴ��ת�Ƶ�����ƿ�У������ж��ݡ�ҡ�ȣ��õ��������Һ�������IJ���ȷ���粻ȷ������ָ���������еĴ����ȱ©֮����_____________________________________________��
��4������˵���������ݲ���Ӧ��ȡ�ľ��巽��_____________________________��
��2��ʵ����������ȡ2mol/LNaOH��Һ�����Ϊ_______________��
��3����������ȡ��NaOH��Һ����С�ձ��м�ˮϡ����ȴ��ת�Ƶ�����ƿ�У������ж��ݡ�ҡ�ȣ��õ��������Һ�������IJ���ȷ���粻ȷ������ָ���������еĴ����ȱ©֮����_____________________________________________��
��4������˵���������ݲ���Ӧ��ȡ�ľ��巽��_____________________________��
��1����2�֣���д����֣�10 mL��Ͳ����������100 mL����ƿ����ͷ�ιܡ�С�ձ�
��2����2�֣�5 mL
��3����3�֣���ȷ����1�֣�ת������Һ��Ӧ������ˮϴ���ձ��Ͳ�����2~3�Σ�����ϴ��ҺҲһͬת������ƿ�С���2�֣�
��4����2�֣�������ƿ��ע������ˮ����̶���1~2 cm�������ý�ͷ�ιܵμ�����ˮ����Һ�� ��Һ��������̶�������
��2����2�֣�5 mL
��3����3�֣���ȷ����1�֣�ת������Һ��Ӧ������ˮϴ���ձ��Ͳ�����2~3�Σ�����ϴ��ҺҲһͬת������ƿ�С���2�֣�
��4����2�֣�������ƿ��ע������ˮ����̶���1~2 cm�������ý�ͷ�ιܵμ�����ˮ����Һ�� ��Һ��������̶�������
��

��ϰ��ϵ�д�
�����Ŀ
 ��˵�����ɣ�����ȷ����ʲ�������
��˵�����ɣ�����ȷ����ʲ�������  ����ԭ�����ʻ�ԭ����������������MnO(OH)2���������KMnO4����Һ�IJ����ǣ�
����ԭ�����ʻ�ԭ����������������MnO(OH)2���������KMnO4����Һ�IJ����ǣ� ��ҺV mL���ʣ�
��ҺV mL���ʣ�