��Ŀ����
�����й�ʵ���������ȷ���ǣ�������
������A��pH��ֽ��������ʪ��
B��Ӧ����40.0g�������ƹ��壻
C�����ý�ͷ�ι���ʢ�е��ۡ�KI��Һ���Թ��еμ���ˮ�������Ƿ������
D�������ų�SO32-�ĸ��ţ�
B��Ӧ����40.0g�������ƹ��壻
C�����ý�ͷ�ι���ʢ�е��ۡ�KI��Һ���Թ��еμ���ˮ�������Ƿ������
D�������ų�SO32-�ĸ��ţ�
����⣺A��pH��ֽ��������ʪ��ֹ����A����
B����500mL����ƿ������Һ��Ӧ����40.0g�������ƹ��壬��B����
C����������Դ��ڵ⣬���ý�ͷ�ι���ʢ�е��ۡ�KI��Һ���Թ��еμ���ˮ�������Ƿ��������C��ȷ��
D�������ų�SO32-�ĸ��ţ�Ӧ�ȼ������ᣬ��������Ȼ���ټ����Ȼ�������D����
��ѡC��
B����500mL����ƿ������Һ��Ӧ����40.0g�������ƹ��壬��B����
C����������Դ��ڵ⣬���ý�ͷ�ι���ʢ�е��ۡ�KI��Һ���Թ��еμ���ˮ�������Ƿ��������C��ȷ��
D�������ų�SO32-�ĸ��ţ�Ӧ�ȼ������ᣬ��������Ȼ���ټ����Ȼ�������D����
��ѡC��
���������⿼�黯ѧʵ�鷽�������ۣ�������ѧ���ķ���������ʵ�����������������Ŀ��飬Ϊ�߿��������ͣ�ע��������ʵ������Լ�ʵ���ע������ѶȲ���

��ϰ��ϵ�д�
 Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�
�����Ŀ
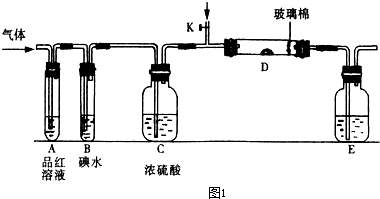



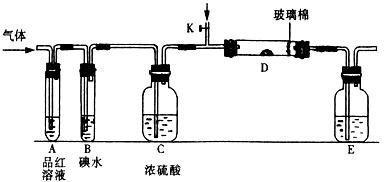
 ��SO2��Cl2��װ��A�й۲쵽�������Ƿ���ͬ�� (���ͬ������ͬ��)����װ��D��װ��
��SO2��Cl2��װ��A�й۲쵽�������Ƿ���ͬ�� (���ͬ������ͬ��)����װ��D��װ�� ��V2O5(����)��ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ ��
��V2O5(����)��ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ �� �� C���������
�� C��������� Һ D��������Һ
Һ D��������Һ l2�뺬X����Һ��Ӧ�����ӷ���ʽ ��
l2�뺬X����Һ��Ӧ�����ӷ���ʽ ��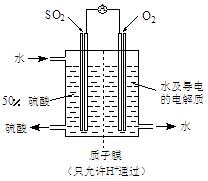


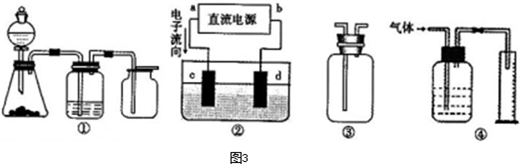
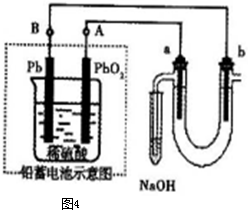
 ��aΪ���⣬dΪ����
��aΪ���⣬dΪ���� 2��NH3��Cl2,��HCl��NO2��
2��NH3��Cl2,��HCl��NO2�� ��װ�â������ڲ����������
��װ�â������ڲ����������