��Ŀ����
(11��)���ʽṹ��Ԫ���������ǻ�ѧ����Ҫ����֪ʶ��ͨ��ѧϰ�ⲿ��֪ʶ�����Զ���ѧԪ�ػ������֪ʶ�����۽ǶȽ�һ���������⡣��A��B��C��D���ֶ�����Ԫ��,���ǵ�ԭ��������A��D��������,��֪A��Bԭ������ͬ�ĵ��Ӳ���,��A��L���������K�������������, Cȼ��ʱ���ֻ�ɫ����, C�ĵ����ڵ�ȼ��������B�ĵ��ʳ�ַ�Ӧ,���Եõ���D������ɫ��ͬ�ĵ���ɫ��̬������,�Ը����������������������:
(1)Ԫ������:A_________�� B__________�� C__________�� D_________��
(2)д��BԪ�������ڱ��е�λ�ã���______���ڣ���________�塣
(3)A��B���γɶ��ֻ�������л���������ЧӦ����һ�ߵĵ���ʽΪ____________
(4)C�����ڵ�ȼ��������B���ʳ�ַ�Ӧ���ù���Ļ�ѧΪ______________���ù��廯��������Ϊ_______________�����ڵ���������________________��
(5) �õ���ʽ��ʾ������C2D���γɹ���_____________________________________��
(1) ̼ �� �� �� (2) �� ��A ��3��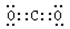
(4) Na2O2 ���ӻ����� ���Ӽ������ۼ�
(5) 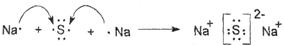
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 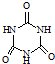 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ�� ��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��һ����Է�������Ϊ78�Ĺ������Ľṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ��
��ҺF����ҺF�ڿ����г��ڷ��÷�����Ӧ��������֮һΪH��H��һ����Է�������Ϊ78�Ĺ������Ľṹ�ͻ�ѧ�������ƣ�����Һ�Ի�ɫ�� NaOH��Һ���յõ���Һ�е�����Ũ���ɴ�С��ϵ�� ��
NaOH��Һ���յõ���Һ�е�����Ũ���ɴ�С��ϵ�� ��