��Ŀ����
1��NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ����1��NaClO��ҺpH��7��ԭ���ǣ������ӷ���ʽ��ʾ��ClO-+H2O?HClO+OH-��
��2������NaClO�������Ʋ⣬��ֽ���м���NaClO��Һ��Ŀ����Ư��ֽ����
��3��ijС��ͬѧ����ͼ��ʾװ��̽������NaClO��KAl��SO4��2��Һ��Ϸ�Ӧ��ʵ�飮��������ƿ�м��뱥��KAl��SO4��2��Һ�����������İ�ɫ��״�������������������ԭ����Al3++3ClO-+3H2O�T3HClO+Al��OH��3�������÷�Ӧ�����ӷ���ʽ��ʾ����
���� ��1��NaClOΪǿ�������Σ�ˮ��ʼ��ԣ�
��2��NaClO����ǿ�����ԣ�������Ư�ף�
��3������ƿ�м��뱥��KAl��SO4��2��Һ�����������İ�ɫ��״������Al3+��ClO-��������ˮ�ⷴӦ��
��� �⣺��1��NaClOΪǿ�������Σ�ˮ��ʼ��ԣ����ӷ���ʽΪClO-+H2O?HClO+OH-���ʴ�Ϊ��ClO-+H2O?HClO+OH-��
��2��NaClO����ǿ�����ԣ�������Ư�ף��ʴ�Ϊ��Ư��ֽ����
��3������ƿ�м��뱥��KAl��SO4��2��Һ�����������İ�ɫ��״������Al3+��ClO-��������ˮ�ⷴӦ�����ӷ���ʽΪAl3++3ClO-+3H2O�T3HClO+Al��OH��3����
�ʴ�Ϊ��Al3++3ClO-+3H2O�T3HClO+Al��OH��3����
���� ���⿼������ˮ��֪ʶ��Ϊ��Ƶ���㣬�����ڻ�ѧ����������Ŀ��飬����������ѧ�����õĿ�ѧ���������ѧϰ�Ļ����ԣ��ѶȲ���ע����ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
�����Ŀ
20�������й����Ӽ����������ȷ���ǣ�������
| A�� | ��ij��Һ�м������ữ��BaCl 2 ��Һ��������ɫ������˵����Һ�к���SO${\;}_{4}^{2-}$ | |
| B�� | ����Һ��ͨ��CO2��������İ�ɫ������˵����Һ�к���Ca2+ | |
| C�� | ���д���Cl-��CO${\;}_{4}^{2-}$��OH-����ɫ��Һ�У��������μ����̪��BaCl2�������ữ��AgNO3��Һ������֤ | |
| D�� | ��ijdz��ɫ��Һ�м���KSCN��Һ����Һ����죬�ټ���H2O2��Һ����Һ��죬˵�����к���Fe2+ |
1�����и�����Һ�п�����������SO42-��SO32-��CO32-��Cl-����õ�һ���ǣ�������
| A�� | BaCl2��Һ������ | B�� | BaCl2��Һ�����ᡢ����ʯ��ˮ | ||
| C�� | BaCl2��Һ��AgNO3��Һ | D�� | BaCl2��Һ�����ᡢƷ����Һ |
6��СҶͬѧ������ʦ��ʵ���ҿ���һ�Լ�ƿ�ı�ǩ��д�š�AgNO30.5mol/L��Һ����0.5mol/L��ʾ���������ǣ�������
| A�� | �ܽ�ȣ�s�� | B�� | ���ʵ���Ũ�ȣ�c�� | C�� | ����������w�� | D�� | Ħ��������M�� |
13�������й�������˵������ȷ���ǣ�������
| A�� | ������ˮ��Һ�ܵ��磬���������ǵ���� | |
| B�� | �����������Ư���ԣ�Һ�Ⱦ��м�ǿ��Ư���� | |
| C�� | ������ʹ��ɫʯ����Һ�ȱ�����ɫ | |
| D�� | ͭ��������ȼ�����ɺ���ɫ�� |
10��ijͬѧ��������þ������ʢ��NH4Cl��Һ���Թ��У��д������ݲ�����Ϊ̽���÷�Ӧԭ������ͬѧ�����������鲢�۲쵽��������ɴ˵ó��Ľ��۲��������ǣ�������
| ѡ�� | ʵ�鼰���� | ���� |
| A | ��ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ���� | ��Ӧ����NH3���� |
| B | �ռ����������岢��ȼ������ʵ���ɫ | ��Ӧ����H2���� |
| C | �ռ������ͬʱ�����Һ��pHΪ8.0 | ��������Һ��MgҲ�ɱ����� |
| D | ��������þ������pHΪ8.6��NaHCO3��Һ�У������ݲ��� | ��������Һ��OH-������Mg |
| A�� | A | B�� | B | C�� | C | D�� | D |
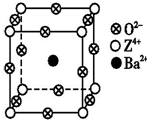 ��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X���л�����Ҫ���Ԫ�أ�X��һ��1��1����̬�⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӣ�
��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X���л�����Ҫ���Ԫ�أ�X��һ��1��1����̬�⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӣ�