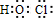��Ŀ����
��2012?�Ӷ���һģ��������ȩΪԭ�ϣ��������µķ�Ӧ��A��B��C��D��E��F��Ϊ�л��������������֪�����ش����⣺
��֪��
��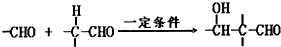
��E�ķ���ʽC12H18O4��B������
��1��A�ķ���ʽΪ��
���л���B��ѧ���ʵ������������
A�����ܷ���������Ӧ���ܷ�����ԭ��Ӧ
B����H2�����ӳɷ�Ӧ���ض��õ�һ�ִ������л���
C���ܷ����Ӿ۷�Ӧ���ɸ߾���
D����ʹBr2��ˮ��Һ��ɫ��1mol���л���ǡ�ú�1mol Br2��Ӧ
��2���ۺܵ͢ķ�Ӧ���ͷֱ�Ϊ����
��3����д���ٵĻ�ѧ��Ӧ����ʽ��
��4����д���Ļ�ѧ��Ӧ����ʽ��
��5������ϵͳ��������F������
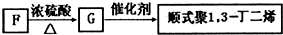
д��G��˳ʽ��1��3-����ϩ�Ļ�ѧ��Ӧ����ʽ��
��6��7.4g C����������Na��Ӧ������H2
��7��A��������ͬ���칹�壬��д�����з�������������A��ͬ���칹�壺
���ܹ�����ˮ�ⷴӦ�����ܹ�����������Ӧ���۷���������

��֪��
��
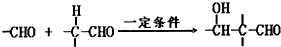
��E�ķ���ʽC12H18O4��B������
��1��A�ķ���ʽΪ��
C4H8O2
C4H8O2
��B�Ľṹ��ʽΪ��CH2=CHCH2CHO
CH2=CHCH2CHO
�����л���B��ѧ���ʵ������������
BD
BD
A�����ܷ���������Ӧ���ܷ�����ԭ��Ӧ
B����H2�����ӳɷ�Ӧ���ض��õ�һ�ִ������л���
C���ܷ����Ӿ۷�Ӧ���ɸ߾���
D����ʹBr2��ˮ��Һ��ɫ��1mol���л���ǡ�ú�1mol Br2��Ӧ
��2���ۺܵ͢ķ�Ӧ���ͷֱ�Ϊ����
��ȥ��Ӧ
��ȥ��Ӧ
���ӳɷ�Ӧ��ԭ��Ӧ
�ӳɷ�Ӧ��ԭ��Ӧ
����3����д���ٵĻ�ѧ��Ӧ����ʽ��
2CH3CHO
CH3CH��OH��CH2CHO
| һ������ |
2CH3CHO
CH3CH��OH��CH2CHO
��| һ������ |
��4����д���Ļ�ѧ��Ӧ����ʽ��
CH3CH��OH��CH2CH2OH+2CH2=CHCH2COOH
2H2O+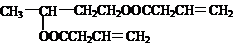
| ŨH2SO4 |
| �� |
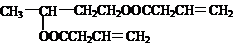
CH3CH��OH��CH2CH2OH+2CH2=CHCH2COOH
2H2O+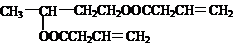
��| ŨH2SO4 |
| �� |
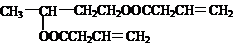
��5������ϵͳ��������F������
1��3-������
1��3-������
��F�������ںϳ����и߾���
д��G��˳ʽ��1��3-����ϩ�Ļ�ѧ��Ӧ����ʽ��
nCH2=CH-CH=CH2
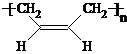
| ���� |
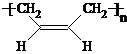
nCH2=CH-CH=CH2
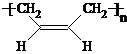
��| ���� |
��6��7.4g C����������Na��Ӧ������H2
1.12
1.12
L����״���£�����7��A��������ͬ���칹�壬��д�����з�������������A��ͬ���칹�壺
���ܹ�����ˮ�ⷴӦ�����ܹ�����������Ӧ���۷���������
HCOOCH2CH2CH3��HCOOCH��CH3��2
HCOOCH2CH2CH3��HCOOCH��CH3��2
��������һ�������£���ȩ��Ӧ����A��A��Ӧ���ɵ�B�ܺ����������ӳɷ�Ӧ����A�к���˫����A��Ũ���������������������·�����Ӧ����B��B�ܺ����������ӳɷ�Ӧ����B����֧������B����˫���������֪����֪��ȩ��֮���ܷ�����Ӧ��������ȩ��ȩ��֮�䷴Ӧ����A����A�Ľṹ��ʽΪ��CH3CH��OH��CH2CHO��A�����������ӳɷ�Ӧ����F����F�Ľṹ��ʽΪ��CH3CH��OH��CH2CH2OH����Ũ���������������������£�A������ȥ��Ӧ����B����B�Ľṹ��ʽΪ��CH2=CHCH2CHO��B�����������ӳɷ�Ӧ����C����C�Ľṹ��ʽΪ��CH3CH2CH2CH2OH����ͭ�����������������£�B��������������D����D�Ľṹ��ʽΪ��CH2=CHCH2COOH����Ũ���������������������£�D��F����������Ӧ����E����E�Ľṹ��ʽΪ��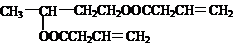 ���ٽ�����ʵ����ʽ��
���ٽ�����ʵ����ʽ��
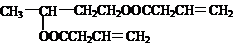 ���ٽ�����ʵ����ʽ��
���ٽ�����ʵ����ʽ������⣺һ�������£���ȩ��Ӧ����A��A��Ӧ���ɵ�B�ܺ����������ӳɷ�Ӧ����A�к���˫����A��Ũ���������������������·�����Ӧ����B��B�ܺ����������ӳɷ�Ӧ����B����֧������B����˫���������֪����֪��ȩ��֮���ܷ�����Ӧ��������ȩ��ȩ��֮�䷴Ӧ����A����A�Ľṹ��ʽΪ��CH3CH��OH��CH2CHO��A�����������ӳɷ�Ӧ����F����F�Ľṹ��ʽΪ��CH3CH��OH��CH2CH2OH����Ũ���������������������£�A������ȥ��Ӧ����B����B�Ľṹ��ʽΪ��CH2=CHCH2CHO��B�����������ӳɷ�Ӧ����C����C�Ľṹ��ʽΪ��CH3CH2CH2CH2OH����ͭ�����������������£�B��������������D����D�Ľṹ��ʽΪ��CH2=CHCH2COOH����Ũ���������������������£�D��F����������Ӧ����E����E�Ľṹ��ʽΪ��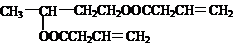 ��
��
��1��A�Ľṹ��ʽΪ��CH3CH��OH��CH2CHO�������ʽΪ��C4H8O2��B�Ľṹ��ʽΪ��CH2=CHCH2CHO��
A��B�к���̼̼˫����ȩ�������Լ��ܷ���������Ӧ���ܷ�����ԭ��Ӧ������ȷ��
B����H2�����ӳɷ�Ӧʱ���ӳ�λ�ò�ͬ�������ﲻͬ���ʴ���
C��B�к���̼̼˫���������ܷ����Ӿ۷�Ӧ���ɸ߾������ȷ��
D����ʹBr2��ˮ��Һ��ɫ��1mol���л�������ܺ�2mol Br2��Ӧ���ʴ���
�ʴ�Ϊ��C4H8O2��CH2=CHCH2CHO��BD��
��2��A�Ľṹ��ʽΪ��CH3CH��OH��CH2CHO��B�Ľṹ��ʽΪ��CH2=CHCH2CHO��C�Ľṹ��ʽΪ��CH3CH2CH2CH2OH��
A��Ӧ����B������ȥ��Ӧ��B��Ӧ����C���ڻ�ԭ��Ӧ��ӳɷ�Ӧ��
�ʴ�Ϊ����ȥ��Ӧ����ԭ����ӳɷ�Ӧ����
��3����һ�������£���ȩ�������Ӽ䷴Ӧ����CH3CH��OH��CH2CHO���÷�Ӧ����ʽΪ��
2CH3CHO
CH3CH��OH��CH2CHO���ʴ�Ϊ��2CH3CHO
CH3CH��OH��CH2CHO��
��4��CH3CH��OH��CH2CH2OH�� CH2=CHCH2COOH��Ũ���������������������·���������Ӧ����������Ӧ����ʽΪ��
CH3CH��OH��CH2CH2OH+2CH2=CHCH2COOH
2H2O+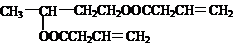 ��
��
�ʴ�Ϊ��CH3CH��OH��CH2CH2OH+2CH2=CHCH2COOH
2H2O+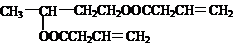 ��
��
��5��F�Ľṹ��ʽΪ��CH3CH��OH��CH2CH2OH��������Ϊ��1��3-��������G��˳ʽ��1��3-����ϩ�ķ�Ӧ����ʽΪ��
nCH2=CH-CH=CH2
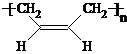 ��
��
�ʴ�Ϊ��1��3-��������nCH2=CH-CH=CH2
 ��
��
��6��7.4g C�����ʵ���=
=0.1mol������������Na��Ӧ���������������=
��22.4L/mol=1.12L���ʴ�Ϊ��1.12��
��7�����ܹ�����ˮ�ⷴӦ��˵���������������ܹ�����������Ӧ��˵������ȩ�����۷��������ʣ�˵����ԭ�Ӳ��Ǵ�������̼ԭ��֮�䣬����A��ͬ���칹��Ľṹ��ʽΪ��HCOOCH2CH2CH3��HCOOCH��CH3��2��
�ʴ�Ϊ��HCOOCH2CH2CH3��HCOOCH��CH3��2��
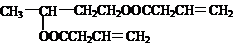 ��
����1��A�Ľṹ��ʽΪ��CH3CH��OH��CH2CHO�������ʽΪ��C4H8O2��B�Ľṹ��ʽΪ��CH2=CHCH2CHO��
A��B�к���̼̼˫����ȩ�������Լ��ܷ���������Ӧ���ܷ�����ԭ��Ӧ������ȷ��
B����H2�����ӳɷ�Ӧʱ���ӳ�λ�ò�ͬ�������ﲻͬ���ʴ���
C��B�к���̼̼˫���������ܷ����Ӿ۷�Ӧ���ɸ߾������ȷ��
D����ʹBr2��ˮ��Һ��ɫ��1mol���л�������ܺ�2mol Br2��Ӧ���ʴ���
�ʴ�Ϊ��C4H8O2��CH2=CHCH2CHO��BD��
��2��A�Ľṹ��ʽΪ��CH3CH��OH��CH2CHO��B�Ľṹ��ʽΪ��CH2=CHCH2CHO��C�Ľṹ��ʽΪ��CH3CH2CH2CH2OH��
A��Ӧ����B������ȥ��Ӧ��B��Ӧ����C���ڻ�ԭ��Ӧ��ӳɷ�Ӧ��
�ʴ�Ϊ����ȥ��Ӧ����ԭ����ӳɷ�Ӧ����
��3����һ�������£���ȩ�������Ӽ䷴Ӧ����CH3CH��OH��CH2CHO���÷�Ӧ����ʽΪ��
2CH3CHO
| һ������ |
| һ������ |
��4��CH3CH��OH��CH2CH2OH�� CH2=CHCH2COOH��Ũ���������������������·���������Ӧ����������Ӧ����ʽΪ��
CH3CH��OH��CH2CH2OH+2CH2=CHCH2COOH
| ŨH2SO4 |
| �� |
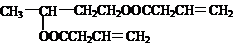 ��
���ʴ�Ϊ��CH3CH��OH��CH2CH2OH+2CH2=CHCH2COOH
| ŨH2SO4 |
| �� |
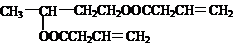 ��
����5��F�Ľṹ��ʽΪ��CH3CH��OH��CH2CH2OH��������Ϊ��1��3-��������G��˳ʽ��1��3-����ϩ�ķ�Ӧ����ʽΪ��
nCH2=CH-CH=CH2
| ���� |
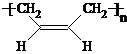 ��
���ʴ�Ϊ��1��3-��������nCH2=CH-CH=CH2
| ���� |
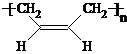 ��
����6��7.4g C�����ʵ���=
| 7.4g |
| 74g/mol |
| 0.1mol |
| 2 |
��7�����ܹ�����ˮ�ⷴӦ��˵���������������ܹ�����������Ӧ��˵������ȩ�����۷��������ʣ�˵����ԭ�Ӳ��Ǵ�������̼ԭ��֮�䣬����A��ͬ���칹��Ľṹ��ʽΪ��HCOOCH2CH2CH3��HCOOCH��CH3��2��
�ʴ�Ϊ��HCOOCH2CH2CH3��HCOOCH��CH3��2��
���������⿼���л�����ƶϣ���ȷ��Ӧǰ���л���ṹ�������ŵı仯�ǽⱾ��ؼ���ע���������Ϣ���з������Ҫע��֪ʶǨ���������������Ѷ��еȣ�

��ϰ��ϵ�д�
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
�����Ŀ
 ��2012?�Ӷ���һģ��A��B��C��D��E����Ԫ�ش������Ұ�ԭ������������ԭ������Ϊ5����������Ȼ������˳���������ң�����˵����ȷ���ǣ�������
��2012?�Ӷ���һģ��A��B��C��D��E����Ԫ�ش������Ұ�ԭ������������ԭ������Ϊ5����������Ȼ������˳���������ң�����˵����ȷ���ǣ�������