��Ŀ����
��ҵ�ͽ�ͨ����ҵ��Ѹ�ٷ�չ�������˿ڵĸ߶ȼ��У��������������������࣬����������д����ŷ��̳����к�����ȣ��Դ�����������ص���Ⱦ�������н���һ�εġ����������ձ�����ʾ�������Ŀ������ܵ�һ���̶ȵ���Ⱦ��һ�о���ѧϰС��������еĿ�����Ⱦ��������������о���
��С��һͬѧ���������ó��������п�����Ⱦ����Ҫԭ��������������֣�
A��ʹ��ʯ��Һ���� B��ȼ�պ���ú C���۳���Ⱦ
��һͬѧ��Ϊ����һ�������ų���ԭ���ǣ�D��
�о���ѧϰС�����������п�����Ⱦ������Ҫԭ������˱���100�������100λ����������ʿ������������ͼ��ʾ��
������Ⱦԭ����������ֲ�ͼ

������ͻ�����ʿ�IJ�ͬ�۵��Ϸ���������Ϊ��������п�����Ⱦ����Ҫ�к��ɷ���
���û�ѧʽ��ʾ��
�ƿ�����Ⱦ�γ����ꡣ�о���ѧϰС��������е���ˮ�����˲����ͷ������ղɼ�ʱ���PHΪ4.82�������ձ��о�2Сʱ���ٴβ��PHΪ4.68���ϳ�һ��ʱ�����PH���ٱ仯���Դˣ���ĺ���������
���о���ѧϰС��ȡ�൱�ڱ�״���µĿ���1.000L����������������������̼����������ȣ�������ͨ��������ˮ����������Һ�м���������Ȼ�����Һ��������ɫ������������ϴ�ӣ�����Ƶ�������Ϊ0.233g��������һʵ���Ŀ���� ��ͨ������õ��Ľ����� ���Զ���������
�ȸ�С��������д�ʩ�Լ��ٿ�����Ⱦ��Ҫ�к��ɷ��ŷ���������Ϊ��������
������ţ�
������Ȼ������ú̿������ȼ�� �ڸĽ�ȼ�ռ��������ú��ȼ��Ч��
�۹�������ʱ��ȼú��¯���̴���ø��� ��ȼú�м�������ʯ��ʯ��ʹ��
��С��һͬѧ���������ó��������п�����Ⱦ����Ҫԭ��������������֣�
A��ʹ��ʯ��Һ���� B��ȼ�պ���ú C���۳���Ⱦ
��һͬѧ��Ϊ����һ�������ų���ԭ���ǣ�D��
�о���ѧϰС�����������п�����Ⱦ������Ҫԭ������˱���100�������100λ����������ʿ������������ͼ��ʾ��
������Ⱦԭ����������ֲ�ͼ

������ͻ�����ʿ�IJ�ͬ�۵��Ϸ���������Ϊ��������п�����Ⱦ����Ҫ�к��ɷ���
���û�ѧʽ��ʾ��
�ƿ�����Ⱦ�γ����ꡣ�о���ѧϰС��������е���ˮ�����˲����ͷ������ղɼ�ʱ���PHΪ4.82�������ձ��о�2Сʱ���ٴβ��PHΪ4.68���ϳ�һ��ʱ�����PH���ٱ仯���Դˣ���ĺ���������
���о���ѧϰС��ȡ�൱�ڱ�״���µĿ���1.000L����������������������̼����������ȣ�������ͨ��������ˮ����������Һ�м���������Ȼ�����Һ��������ɫ������������ϴ�ӣ�����Ƶ�������Ϊ0.233g��������һʵ���Ŀ���� ��ͨ������õ��Ľ����� ���Զ���������
�ȸ�С��������д�ʩ�Լ��ٿ�����Ⱦ��Ҫ�к��ɷ��ŷ���������Ϊ��������
������ţ�
������Ȼ������ú̿������ȼ�� �ڸĽ�ȼ�ռ��������ú��ȼ��Ч��
�۹�������ʱ��ȼú��¯���̴���ø��� ��ȼú�м�������ʯ��ʯ��ʹ��
�Ż�����β����Ⱦ�� SO2����2�֣�
����ˮ�е�H2SO3�������е�����������H2SO4��2�֣�
�Dzⶨ�����ж�����������������2�֣��������ж���������������Ϊ2.24%��2�֣�
�Ȣ٢ڢ� ��2�֣�
����ˮ�е�H2SO3�������е�����������H2SO4��2�֣�
�Dzⶨ�����ж�����������������2�֣��������ж���������������Ϊ2.24%��2�֣�
�Ȣ٢ڢ� ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 2Ni��OH��2��
2Ni��OH��2��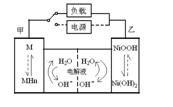

 ��K��һ��ֻ���¶��йصij�������Ϊ��ѧƽ�ⳣ����
��K��һ��ֻ���¶��йصij�������Ϊ��ѧƽ�ⳣ����