��Ŀ����
��չ��϶�������ʵʩ���ܼ��ŵ���Ҫ��ʩ֮һ����϶����������õ綯������ȼ������߽���ƶ����֡��������»����ʱ���綯���ṩ�ƶ��������������͵����ģ���ɲ��������ʱ�綯�����ڳ��״̬�Խ�ʡ�ܺġ�
��1����϶���������ȼ��������Ϊȼ�ϣ����ͣ�������C8H18�ƣ���������ַ�Ӧ��ÿ����1molˮ��������569��1kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
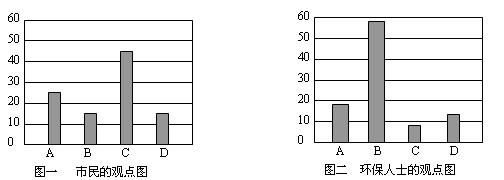
��2����϶������ĵ綯��Ŀǰһ��ʹ�õ��������أ������ز������Ļ�����Ϊ�����������������M��ʾ��Ϊ��������Һ����ҪΪKOH��Ϊ���Һ�������س�ŵ�ԭ������ͼ�����ܷ�Ӧʽ�ǣ�
H2+2NiOOH 2Ni��OH��2��
2Ni��OH��2��
����������Ϣ�жϣ���϶��������»����ʱ���ҵ缫��Χ��Һ��pH��
������������䡱��С�������õ缫�ĵ缫��ӦʽΪ ����ɲ��������ʱ�缫�ĵ缫��ӦΪ ��
��3������β���е�һ����̼�Ǵ�����Ⱦ���ͨ�����·�Ӧ������Ũ�ȣ�
��g������֪��ƽ��ʱ�����ʵ�Ũ�ȹ�ϵ��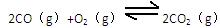
��֪��ƽ��ʱ�����ʵ�Ũ�ȹ�ϵ��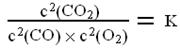 ��K��һ��ֻ���¶��йصij�������Ϊ��ѧƽ�ⳣ����
��K��һ��ֻ���¶��йصij�������Ϊ��ѧƽ�ⳣ����
��ij�¶��£������������н���������Ӧ�������и����ʵ���ʼŨ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д���еĿո�
����ͬ�¶��£�ij����β����CO��CO2��Ũ�ȷֱ�Ϊ1��0��10��5 mol/L��1��0��10��4mol/L������������������������һ����ȼ�������ϲ���O2��ʹ��Ũ�ȱ���Ϊ1��0��10��4mol/L��������β����CO��Ũ��Ϊ ��
��1����϶���������ȼ��������Ϊȼ�ϣ����ͣ�������C8H18�ƣ���������ַ�Ӧ��ÿ����1molˮ��������569��1kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��2����϶������ĵ綯��Ŀǰһ��ʹ�õ��������أ������ز������Ļ�����Ϊ�����������������M��ʾ��Ϊ��������Һ����ҪΪKOH��Ϊ���Һ�������س�ŵ�ԭ������ͼ�����ܷ�Ӧʽ�ǣ�
H2+2NiOOH
 2Ni��OH��2��
2Ni��OH��2��
����������Ϣ�жϣ���϶��������»����ʱ���ҵ缫��Χ��Һ��pH��
������������䡱��С�������õ缫�ĵ缫��ӦʽΪ ����ɲ��������ʱ�缫�ĵ缫��ӦΪ ��
��3������β���е�һ����̼�Ǵ�����Ⱦ���ͨ�����·�Ӧ������Ũ�ȣ�
��g������֪��ƽ��ʱ�����ʵ�Ũ�ȹ�ϵ��
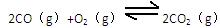
��֪��ƽ��ʱ�����ʵ�Ũ�ȹ�ϵ��
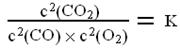 ��K��һ��ֻ���¶��йصij�������Ϊ��ѧƽ�ⳣ����
��K��һ��ֻ���¶��йصij�������Ϊ��ѧƽ�ⳣ������ij�¶��£������������н���������Ӧ�������и����ʵ���ʼŨ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д���еĿո�
| ������ �� | c��CO��/ mol/L | c��O2��/ mol/L | c��CO2��/ mol/L | v��������v���棩�ıȽ� |
| �� | 2��0��10��4 | 4��0��10��4 | 4��0��10��2 | v������= v���棩 |
| �� | 3��0��10��4 | 4��0��10��4 | 5��0��10��2 | |
��1��C8H18��l��+25/2 O2 =" 8" CO2��g��+ 9 H2O��g������H= ��5121��9 kJ/mol
��2������ NiOOH + H2O+e��= Ni��OH��2 + OH�� 2 H2O + 2e��= H2 + 2OH��
��3���� v������> v���棩 �� 1��1��10��6
��2������ NiOOH + H2O+e��= Ni��OH��2 + OH�� 2 H2O + 2e��= H2 + 2OH��
��3���� v������> v���棩 �� 1��1��10��6
��

��ϰ��ϵ�д�
�����Ŀ