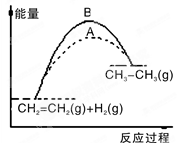
��Ŀ����
��֪һ�������¶���1 mol���л�ѧ��������̬ԭ����Ҫ���յ��������£�H��H��436 kJ��Cl��Cl��243 kJ��H��Cl��431 kJ�����������Ȼ�ѧ����ʽ�������ȷ���� (����)��
| A��2HCl(g)=H2(g)��Cl2(g)�ķ�Ӧ�Ȧ�H<0 |
| B��H(g)��Cl(g)=HCl(g)����H����431 kJ��mol��1 |
| C����ͬ�����£�H2(g)��Cl2(g)=2HCl(g)�ڹ��պ͵�ȼ�����µĦ�H��� |
| D��H2(g)��Cl2(g)=2HCl(g)����H����183 kJ |
��C
����

 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д���֪�� Cu ��s��+2H+��aq����Cu2+��aq��+ H2��g�� ��H 1
2H2O2��l����2H2O��l�� + O2 ��g�� ��H 2
2H2��g�� + O2��g����2H2O��l�� ��H 3
��Ӧ Cu ��s��+H2O2��l��+2H+ ��aq����Cu2+��aq��+ 2H2O��l�� �ġ�H��
A����H����H 1+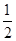 ��H 2+ ��H 2+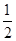 ��H 3 ��H 3 | B����H����H 1+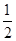 ��H 2- ��H 2-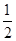 ��H 3 ��H 3 |
| C����H����H 1+2��H 2+2��H 3 | D����H��2��H 1+��H 2+��H 3 |
��֪��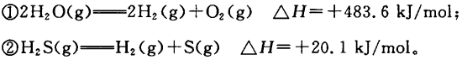
�����ж���ȷ���� ( )
| A��������ȼ���ȣ���H="-241.8" kJ/mol |
| B����ͬ�����£����ȼ��1 mol H2(g)��1 molS(g)�Ļ����ȳ��ȼ��1 molH2S (g)���ȶ�20.1 kJ |
| C���ɢ٢�֪��ˮ�����ȶ���С������ |
| D�����������ɹ�̬��H������ |
��֪����CO��g����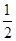 O2��g��=CO2��g������H����283.0 kJ��mol��1
O2��g��=CO2��g������H����283.0 kJ��mol��1
��H2��g����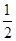 O2��g��=H2O��g�� ��H����241.8 kJ��mol��1
O2��g��=H2O��g�� ��H����241.8 kJ��mol��1
��CO��g����H2O��g��=H2��g����CO2��g���Ħ�HΪ
| A��+41.2 kJ?mol-1 | B����41.2 kJ?mol-1 | C��+82.4kJ?mol-1 | D����524.8 kJ?mol-1 |
���з�Ӧ���ڷ��ȷ�Ӧ���ǣ� ��
��ϡ����������������Һ��Ӧ
��п��ϡ����ķ�Ӧ
����ʯ�ұ����ʯ�ҵķ�Ӧ
����������������Ȼ�茶����Ϸ�Ӧ
��ʯ��ʯ��������
�����ȵ�̿��CO2��Ӧ
������O2��ȼ�շ�Ӧ
| A���٢ڢۢ� | B���٢ڢۢ� |
| C���٢ڢݢޢ� | D���ܢ� |
100 g̿��ȼ�����������У�COռ ��CO2ռ
��CO2ռ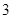 ����C(s)��
����C(s)��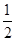 O2(g)=CO(g)����H����110.35 kJ��mol��1��CO(g)��
O2(g)=CO(g)����H����110.35 kJ��mol��1��CO(g)��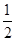 O2(g)=CO2(g)����H����282.57 kJ��mol��1������Щ̿����ȫȼ�������ʧ�������� (����)��
O2(g)=CO2(g)����H����282.57 kJ��mol��1������Щ̿����ȫȼ�������ʧ�������� (����)��
| A��784.92 kJ | B��2 489.44 kJ | C��1 569.83 kJ | D��3 274.3 kJ |
��֪C2H5OH��g����3O2��g��=2CO2��g����3H2O��g�� ��H1��-a kJ/mol
C2H5OH��g��=C2H5OH��l�� ��H2��-b kJ/mol
H2O��g��=H2O��l�� ��H3��-c kJ/mol
��ʹ92 g�ƾ�Һ����ȫȼ�գ����ָ������£���ų�����������λkJ��Ϊ�� ��
| A��4a��4b��4c | B��2a-2b+6c |
| C��2a��2b��2c | D��2a��6b��2c |
(2013���㶫��ɽһģ��11)���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ����(˫ѡ)(����)��
| A����֪2H2(g)��O2(g)===2H2O(g)����H����483.6 kJ��mol��1����������ȼ����Ϊ241.8 kJ��mol��1 |
| B����֪NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57.3 kJ��mol��1����40.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�С��57.3 kJ������ |
| C����֪2C(s)��2O2(g)===2CO2(g)����H��a��2C(s)��O2(g)===2CO(g)����H��b����a>b |
| D����֪C(ʯī��s)===C(���ʯ��s)����H>0����ʯī�Ƚ��ʯ�ȶ� |