��Ŀ����
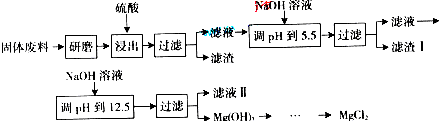
��2009?�㶫�����쳣�Ӱ����ֳ���������ζ����ȡ����������������ζ�ijɷ��к������»���������������ۻ�ʮ��������5��9һʮһ������������ʮ�����ȩ���߸�ȩ������˵����ȷ���ǣ�������
�����������л�����������л����к���ʮ�������µ�̼�����ü��ұ����缺�����ɹ����ֱ��ʾ��10�����������ֱ�ʾ��Ȼ�������Ӧ�Ĺ�������������������ϩ����Ȳ�������ᡢȩ�ࡢ����Ⱦ������л�����д����Ȼ������������ԣ�Ϊ���Ի���������Ȼ��IJ������Ի��������������ֻ��̼������Ԫ�أ�������Ԫ�أ����ᡢȩ�����к���̼��������Ԫ�ص�֪ʶ�������⣮
����⣺A���������к���8��̼ԭ�ӣ��������к���9��̼���ۻ�ʮ�����к���12��̼����5��9һʮһ���������к���11��̼����ʮ�����к���18��̼��ȩ�к���6��̼���߸�ȩ����7��̼���١��ڡ��ޡ��߷�����̼ԭ����С��10���ۡ��ܡ��ݷ�����̼ԭ��������10����A��ȷ��
B�������ᡢ���������������൱ȻҲ�����л����B����
C�������ᡢ�����������ᣬΪ���Ի�����ۻ�ʮ���������ڴ�����ʮ�����������������������ᣬ�ʲ�Ϊ���Ի�����������ƿ��жϣ���C��ȷ��
D���ޢ�����ȩ�����ʣ�����-CHO����Ȼ������Ԫ�أ���D����
��ѡAC��
B�������ᡢ���������������൱ȻҲ�����л����B����
C�������ᡢ�����������ᣬΪ���Ի�����ۻ�ʮ���������ڴ�����ʮ�����������������������ᣬ�ʲ�Ϊ���Ի�����������ƿ��жϣ���C��ȷ��
D���ޢ�����ȩ�����ʣ�����-CHO����Ȼ������Ԫ�أ���D����
��ѡAC��
�������������л���ķ��࣬��������ϩ����Ȳ�������ᡢȩ�ࡢ����ȣ�����ԭ��Ϊ����ʮ�������µ�̼�����ü��ұ����缺�����ɹ����ֱ��ʾ��10�����������ֱ�ʾ��������Ӧ�Ĺ����ţ������������������������Ի�������ᡢȩ�ࡢ���ຬ����ԭ�ӵ�֪ʶ�㣮

��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
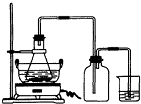 ��2009?�㶫ģ�⣩�̷���FeSO4?7H2O������Ҫ�Ļ�ѧ�����Լ�����ҵ�ϳ����û�е�ӹ���ҵ�����ķ���мΪԭ���Ʊ�����ش��������⣺
��2009?�㶫ģ�⣩�̷���FeSO4?7H2O������Ҫ�Ļ�ѧ�����Լ�����ҵ�ϳ����û�е�ӹ���ҵ�����ķ���мΪԭ���Ʊ�����ش��������⣺