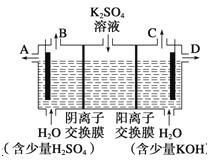
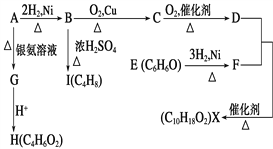
��Ŀ����
����Ŀ��Ŀǰ�������϶���õ�������Ȼ��Ƶķ��������������ƣ�2NaCl(����) ![]() 2Na��Cl2������֪A��B��C��D��E������ת����ϵ��
2Na��Cl2������֪A��B��C��D��E������ת����ϵ��
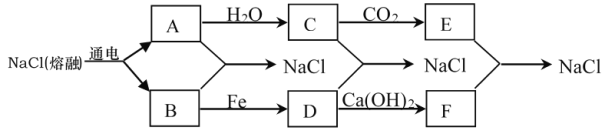
��1��д��A��B����NaCl�Ļ�ѧ����ʽ��_____________________________________��
��2��д����ѧʽ��C______________��D_____________��
��3����ҵ�����г�����B��Ca(OH)2��Ӧ���Ʊ�Ư�ۣ�Ư�۵���Ҫ�ɷ���_______________________________����д��ѧʽ��
��4������AͶ��ʢ��D����Һ�У���Һ�г���________________(�������ɫ)�������ù�����������Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
���𰸡�2Na+Cl2![]() 2NaCl NaOH FeCl3 CaCl2��Ca(ClO)2 ���ɫ 2Na+2H2O=2NaOH+H2�� FeCl3+3NaOH=Fe(OH)3��+3NaCl
2NaCl NaOH FeCl3 CaCl2��Ca(ClO)2 ���ɫ 2Na+2H2O=2NaOH+H2�� FeCl3+3NaOH=Fe(OH)3��+3NaCl
��������
�����̿�֪��A��ˮ��Ӧ����C����C�������̼��Ӧ����AΪNa��CΪNaOH��EΪNa2CO3��BΪCl2��DΪFeCl3��FΪCaCl2�����Ԫ�ػ��ϼ�֪ʶ����ѧ���������
��1��AΪNa��BΪCl2������NaCl�Ļ�ѧ����ʽ��2Na+Cl2![]() 2NaCl��
2NaCl��
��2���ɷ�����֪�����ʵĻ�ѧʽ��CΪNaOH��DΪFeCl3��
��3����ҵ����ȡƯ�ۣ���Ӧ�ķ���ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��Ư�۵���Ҫ�ɷ���CaCl2��Ca(ClO)2��
��4������AΪNaͶ��ʢ��DΪFeCl3����Һ�У�A��ˮ��Ӧ����NaOH��2Na+2H2O=2NaOH+H2�� ���ٷ���3NaOH+FeCl3�TFe��OH��3��+3NaCl����Һ�г��ֺ��ɫ������

 ������������ϵ�д�
������������ϵ�д�