��Ŀ����
����Ŀ������ͼװ�ý���Ũ�����ľ̿��Ӧ��ʵ�飬��������CO2��SO2�����������ɡ�

�ش��������⣺
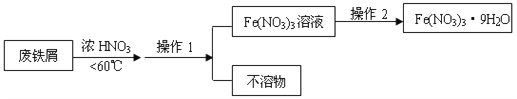
��1������a������Ϊ_________��Aװ���з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��2�����߿�����һ��������ָ��______________________��
װ��B��������___________________________________________��
��3��װ��C������KMnO4��Һ���ڳ�ȥSO2����Ŀ����________��������ţ�
�ף���ֹSO2����CO2�ļ��� ���� �ң�����CO2���� ������������SO2
��4��װ��E��������_______________________________��E�з�����Ӧ�����ӷ���ʽΪ________________________________________________��
���𰸡� ��Һ©�� C+2H2SO4��Ũ�� ![]() CO2��+2SO2��+2H2O C�е�����Ӧ�ó���̳� ��֤��SO2���ɡ� �� ����ʯ��ˮ����� CO2+Ca2++2OH��=CaCO3��+H2O��
CO2��+2SO2��+2H2O C�е�����Ӧ�ó���̳� ��֤��SO2���ɡ� �� ����ʯ��ˮ����� CO2+Ca2++2OH��=CaCO3��+H2O��
�������������������1������a�Ƿ�Һ©����̿��Ũ���ᷴӦ���ɶ�����̼����������ˮ��
��2��ϴ��ƿӦ���dz��ܽ������̹ܳ���������������ʹƷ����ɫ��
��3��������̼������������ʹ����ʯ��ˮ����ǡ�
��4��������̼��ʹ����ʯ��ˮ����ǡ�
��������1������a�Ƿ�Һ©����̿��Ũ���ᷴӦ���ɶ�����̼����������ˮ������ʽΪC+2H2SO4��Ũ�� ![]() CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O��
��2�����߿��ڵĴ�����C�е�����Ӧ�ó���̳�������������ʹƷ����ɫ��װ��B����������֤��SO2���ɡ�
��3��������̼������������ʹ����ʯ��ˮ����ǣ�װ��C������KMnO4��Һ���ڳ�ȥSO2����Ŀ���Ƿ�ֹSO2����CO2�ļ��顣
��4��E�г���ʯ��ˮ����ǣ���Ӧ���ӷ���ʽCO2+Ca2++2OH��=CaCO3��+H2O��

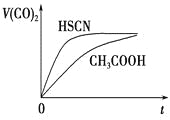
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�����Ŀ���±��dz�����ijЩһԪ����ĵ��볣����
���� | HCN | HF | CH3COOH | HNO2 |
���볣�� | 4.9��10��10 | 7.2��10��4 | 1.8��10��5 | 6.4��10��6 |
��0.1 mol��L��1��������Һ�У�pH��С����
A. HCN B. HF C. CH3COOH D. HNO2
����Ŀ������ʵ�鷽���У������ﵽ��Ӧʵ��Ŀ�ĵ���
A | B | C | D | |
�� �� |
|
|
|
|
Ŀ �� | ��֤��ͬ�����Ի�ѧ��Ӧ���ʵ�Ӱ�� | ��֤ʯ���ͷֽ�IJ����к������������ʲ�ͬ���� | ��ȥ��ϩ�����������е���ϩ | �Ƚ�Cl2��I2�������� |
A. A B. B C. C D. D