��Ŀ����
NaClO2��һ����Ҫ��ɱ������������һ����������ԭ�����£�
��2NaClO3+SO2+H2SO4==2ClO2+2NaHSO4
��2ClO2+NaCl 2NaClO2+Cl2
2NaClO2+Cl2
�ش��������⣺
��1��NaClO2�е�Cl �Ļ��ϼ�__________��
��2������Ӧ�ٸ�Ϊ���ӷ�Ӧ����ʽ_______________________��
��3�������Ӧ�ڵĵ���ת�Ƶķ������Ŀ_________________________��
��4�����ڵ���ʳ��ˮ���ȳ�ȥ����Ca2+��Mg2+��SO42-�����ʣ����Ӳ���ʱ�����μ�����Լ�˳��ΪNaOH��_________��_________��ַ�Ӧ������һ�����˳�ȥ��
��5����������β����������ClO2��(�������Ⱦ���ǿ������)������H2O2������Һ���ճ�ȥ���÷�Ӧ�е���������Ϊ_________��
��6��KClO2��Cl2���ܽ���Ʒ�ˮ��CN- ����Ϊ�������ʣ���������ԭΪCl-��������CN- ��ͬ����Ʒ�ˮ����Cl2�����ʵ�����KClO2__________����
��ϰ��ϵ�д�
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
�����Ŀ


 �ı�ʾ��ȷ����( )
�ı�ʾ��ȷ����( )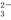 )��c(
)��c( HCO
HCO )
)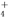 )>c(OH��)
)>c(OH��) Һ
Һ